Piramal Imaging's partner in Canada has received marketing authorization from Health Canada to begin commercial production and distribution of its Neuraceq radiotracer for diagnosing Alzheimer's disease.
Isologic Innovative Radiopharmaceuticals received a notice of compliance from Health Canada stating that it could begin production; previously, use of the agent was limited to investigational purposes.
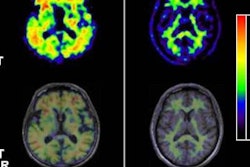
Neuraceq previously received approval from the U.S. Food and Drug Administration (FDA) and several countries in the European Union and Asia. When used in combination with PET, it can identify beta-amyloid plaques in the brain, which can be biomarkers for Alzheimer's disease.