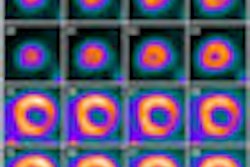
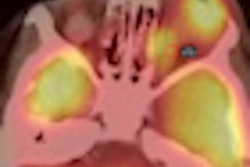
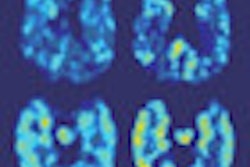
Bracco Diagnostics this week resumed shipments of its CardioGen-82 cardiac PET radiopharmaceutical after the U.S. Food and Drug Administration (FDA) approved revised labeling for the product.
CardioGen-82 will now include a boxed warning and enhanced testing information to help minimize the risk for exposure to unintended levels of strontium radiation.
The revised labeling establishes new alert limits for strontium-82 (Sr-82) and strontium-85 (Sr-85) levels in the generator eluate. If the eluate reaches these alert limits, additional Sr-82 and Sr-85 eluate testing is required. Previously, according to the agency, eluate testing was done only once a day.
With the revised labeling, the testing is still done daily, but additional tests are performed that day if a generator eluate has reached the alert limit. The additional daily testing is necessary to promptly identify excess strontium levels, which indicate that the generator expiration is approaching.
The instructions also include new information to identify when a generator is expired and must no longer be used.
In a February 14 letter to its customers, Bracco announced plans to resume CardioGen-82 production and generator availability in February and March. In addition, customer training and education are under way to inform users of the revised product prescribing information and quality control procedures.
In July 2011, Bracco voluntarily recalled CardioGen-82 after three patients at two sites were exposed to more radiation than typically associated with a CardioGen-82 scan.
Earlier this month, the FDA confirmed that improper use of the radiopharmaceutical by the two clinical sites caused the additional radiation. The agency's investigation also found no fault with Bracco's product or manufacturing process.