GE Healthcare is touting the results of two studies that suggest the company's investigational imaging agent F-18 flutemetamol could combine with other diagnostic tools to evaluate neurodegenerative conditions such as Alzheimer's disease.
Flutemetamol is currently in phase III development for the detection of beta amyloid, which has been linked to Alzheimer's. The results were presented at the annual Human Amyloid Imaging (HAI) meeting in Miami.
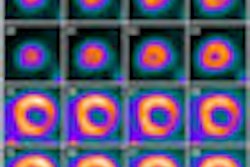
One study looked at patients suspected of having normal pressure hydrocephalus (NPH), a progressive condition associated with dementia, gait abnormalities, and urinary incontinence, who were undergoing shunt replacement. Researchers correlated flutemetamol uptake with histopathological tissue biopsies for beta amyloid in vivo.
Flutemetamol uptake demonstrated strong agreement with histopathology in subjects with NPH, regardless of timing and exam sequence. Flutemetamol PET uptake achieved 100% sensitivity and specificity with histopathology in a selected subset of subjects with NPH.
In the second study, researchers evaluated flutemetamol uptake and structural MRI in healthy volunteers and patients with a clinical diagnosis of Alzheimer's or mild cognitive impairment.
In a subset of patients with mild cognitive impairment, increased flutemetamol uptake and decreased hippocampal volume were seen in subjects with progressive mild cognitive impairment, compared to individuals with stable mild cognitive impairment. The hippocampus region of the brain is involved in memory and other cognitive functions.
Jonathan Allis, molecular imaging PET segment leader for GE, said the results support the potential role of flutemetamol to detect amyloid deposits in the brain and could help physicians make a more accurate and earlier diagnosis of Alzheimer's disease.