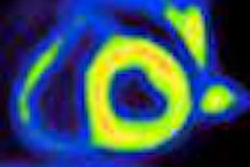
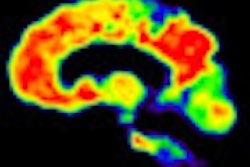
Using PET imaging with the radiopharmaceutical carbon-11-labeled Pittsburgh Compound B (C-11 PiB), researchers found that treatment with the antibody gantenerumab appeared to reduce amyloid levels in the brains of Alzheimer's patients, according to a report published online October 10 in Archives of Neurology.
Gantenerumab is a human anti-amyloid-beta monoclonal antibody currently in clinical development for the treatment of Alzheimer's disease by F. Hoffmann-La Roche Neuroscience and GE Healthcare. In previous studies, gantenerumab has bound in vivo to amyloid-beta plaques in mice. Five months of treatment with gantenerumab significantly decreased the amyloid plaque load in the mice.
The ability to reduce amyloid-beta plaque levels in the brain could help decrease or slow the progression of Alzheimer's disease and dementia, as amyloid plaque is associated with the advance of both neurological conditions.
In this study, two consecutive cohorts of patients with mild-to-moderate Alzheimer's disease received two to seven infusions of intravenous gantenerumab (60 or 200 mg) or placebo every four weeks. In addition, brain tissue from two patients with Alzheimer's was obtained during tumor surgery and was coincubated with gantenerumab as an ex vivo study (Arch Neurol, October 10, 2011).
The levels of amyloid-beta in the brain were measured using C-11 PiB with PET (Ecat Exact HR+, Siemens Healthcare). C-11 PiB binds to amyloid in neural tissues and can be used to evaluate changes in amyloid levels.
PiB-PET was instrumental in a recent study that concluded passive immunization can reduce brain amyloid levels in vivo after 18 months of treatment, according to the authors. The approach was also less likely to induce severe neuroinflammation. PiB-PET has also demonstrated that it can be used to detect high concentrations of amyloid plaques during the early stages of Alzheimer's disease.
In the current study, lead author Dr. Susanne Ostrowitzki, from Hoffmann-La Roche, and colleagues determined the reduction in amyloid-beta plaque was directly related to the size of the gantenerumab dose. The mean percent change from baseline difference relative to placebo in cortical brain amyloid level was -15.6% for patients receiving 60 mg of gantenerumab and -35.7% for those receiving 200 mg of gantenerumab.
The efficacy of gantenerumab was also seen in the mean percent change from baseline compared to total PiB signal, at 11% for the placebo group, 2.1% for the 60-mg group, and -9.4% for the 200-mg group.
"Our study demonstrates that two to seven months of treatment with gantenerumab led to dose-dependent amyloid reduction in the brains of patients with Alzheimer's disease," the authors wrote. "Additionally, our findings in the placebo-treated patients support previous reports indicating that amyloid load continues to increase in many patients with mild-to-moderate Alzheimer's disease."
It is still unclear whether any reduction in brain amyloid levels will translate into clinical efficacy, Ostrowitzki and colleagues added. A phase II clinical trial is under way to investigate whether a clinical benefit can be achieved in gantenerumab-treated patients with Alzheimer's disease.
| The research is supported by F. Hoffmann-La Roche. Several authors, including Ostrowitzki, are full-time employees of Roche/F. Hoffmann-La Roche. Study co-author Lennart Thurfjell, PhD, is a full-time employee of GE Healthcare. |