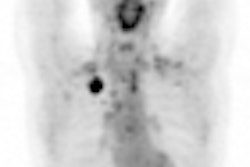
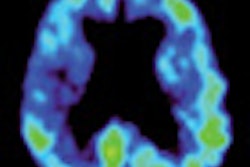
GE Healthcare has begun selling its DaTscan (ioflupane I-123 injection) imaging agent to help physicians evaluate patients with suspected Parkinsonian syndromes, such as Parkinson's disease.
DaTscan was approved by the U.S. Food and Drug Administration (FDA) in January 2011 after going through the agency's priority review process, and it's now available at more than 80 hospitals across the U.S. It has been available in Europe since 2000 and has been used in approximately 300,000 patients in 34 countries.
DaTscan is an adjunct to other diagnostic evaluations to help differentiate essential tremor from tremors due to Parkinsonian syndromes. GE noted that the effectiveness of DaTscan as a screening or confirmatory test and for monitoring disease progression or response to therapy has not been established.