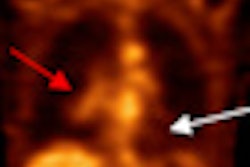
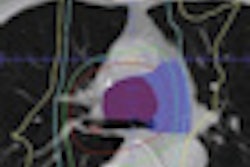
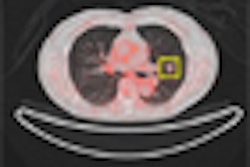
Although FDG-PET/CT is useful for monitoring patients with bone metastases from breast cancer, additional prospective studies are needed to define the roles of FDG-PET/CT and circulating tumor cells in measuring the disease, according to a new study in the August issue of the Journal of Nuclear Medicine.
Circulating tumor cells (CTCs) are cells that can break from a tumor, travel through the bloodstream, and eventually become tumors in organs such as the breast, liver, or colon. The presence of CTCs before treatment is a predictor of progression-free survival and overall survival in patients with metastatic breast cancer.
Researchers from M. D. Anderson Cancer Center at the University of Texas in Houston found that CTC counts at follow-up agreed with FDG-PET/CT assessment in 78% of the study's patients. In addition, FDG-PET/CT findings and follow-up CTC counts were "significantly associated with both progression-free survival and overall survival."
The lead author of the study was Ugo De Giorgi, MD, from the department of breast medical oncology at M. D. Anderson (JNM, August 2010, Vol. 51:8, pp. 1213-1218).
CTC numbers
Another study by De Giorgi and colleagues, published earlier this year in the Annals of Oncology, found that the presence of extensive bone metastases as detected by FDG-PET/CT was associated with increased CTC numbers in metastatic breast cancer (Ann Oncol, January 2010, Vol. 21:1, pp. 33-39).
"Specifically, we showed that CTC numbers were higher in patients with bone metastases than in those with no bone lesions and higher in patients with three or more old metastases than in those with fewer bone lesions," the authors wrote.
With that as the foundation, the authors launched a new study to compare the predictive significance of FDG-PET/CT and CTC counts in patients with bone metastases from breast cancer treated with standard systemic therapy.
The retrospective study reviewed the M. D. Anderson breast medical oncology database and identified patients who had received systemic treatment for bone metastases from breast cancer between December 2004 and May 2008, as well as patients with intrathoracic lymph node or chest wall metastases in addition to bone metastases. Patients with visceral metastases were excluded.
All 55 patients enrolled in the study underwent FDG-PET/CT scans (Discovery ST, STE, or RX, with 8-, 16-, or 64-slice CT, GE Healthcare, Chalfont St. Giles, U.K.) and CTC evaluation within three weeks before starting a new treatment.
Twenty-six patients received systemic therapies for bone metastases only, and 29 patients received treatment for bone metastases and intrathoracic lymph node or chest wall metastases after undergoing both CTC and PET/CT evaluation.
Thirty-six patients (65%) had received prior treatment for metastatic breast cancer with hormone therapy (23 cases), chemotherapy with or without hormone therapy (nine cases), or HER2-targeted therapies with chemotherapy or hormone therapy (four cases). Nineteen patients (35%) had newly diagnosed metastatic breast cancer.
Disease progression
In their analysis, the researchers found CTC count at follow-up agreed with the FDG-PET/CT assessment for nonprogressive disease or progressive disease in 43 patients (78%).
Among the remaining patients, four patients (33%) with fewer than five circulating tumor cells per 7.5 mL of blood at follow-up were found to have evidence of progressive disease by FDG-PET/CT, compared with eight patients (67%) with persistent CTCs (≥ 5 CTCs/7.5 mL of blood) at follow-up who did not have evidence of progressive disease.
In all 55 patients, the mean for progression-free survival was 10.5 ± 7.2 months, ranging from two to 34 months. The mean overall survival was 18.1 ± 6.7 months, with a range of three to 37 months.
At the time of analysis, seven patients (13%) were considered progression-free, with an average follow-up time of 18 months, ranging from 10 to 34 months. Eighteen patients (33%) had died.
Follow-up CTC counts and FDG-PET/CT assessment for nonprogressive or progressive disease were found to be significantly associated with both progression-free and overall survival. The researchers also concluded that baseline CTC count was "not a significant predictor for either progression-free survival or overall survival."
The group also found that median progression-free survival was 13 months in patients with both < 5 CTCs/7.5 mL of blood and FDG-PET/CT nonprogression. The median progression-free survival was six months in patients with < 5 CTCs/7.5 mL of blood or FDG-PET/CT nonprogression, but not both, while the median progression-free survival was five months in patients with neither < 5 CTCs nor FDG-PET/CT nonprogression.
"The multivariate analysis indicated that FDG-PET/ CT was the only predictive sign," De Giorgi and colleagues wrote. "However, the combination of FDG-PET/CT and CTC might be a useful tool to monitor response to therapy in patients without measurable extraosseous disease, especially in patients with elevated CTC at baseline."
They also noted that the discordance of FDG-PET/CT assessment and CTC count "needs to be evaluated in a prospective study to determine the value of FDG-PET/CT and CTC individually and in combination."
"A prospective study could validate the benefit of these two approaches used separately and in combination in determining prognosis, monitoring response, and establishing bone-dominant disease as a tumor response-measurable disease," they wrote.
By Wayne Forrest
AuntMinnie.com staff writer
August 26, 2010
Related Reading
Investigational PET agent shows promise for breast cancer, April 30, 2010
Whole-body PET better for palpable breast cancer, May 2, 2008
Whole-body FDG-PET of little use in breast cancer staging, November 26, 2006
Preop FDG-PET spots axillary involvement in breast cancer, may obviate biopsy, August 21, 2006
Dual-time-point breast PET increases diagnostic accuracy, December 2, 2005
Copyright © 2010 AuntMinnie.com