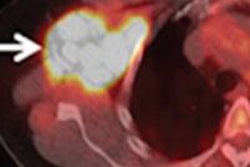
Medical isotope developer Advanced Medical Isotope (AMIC) is continuing to move forward on its long odyssey toward receiving U.S. Food and Drug Administration (FDA) clearance for its yttrium-90 (Y-90) RadioGel brachytherapy cancer device.
AMIC CEO Jim Katzaroff and the company's lead scientific advisory team met with FDA representatives last week to discuss the next steps, expectations, and required testing to progress toward marketing clearance for Y-90 RadioGel, according to the firm. After obtaining all appropriate data and benchmarks required by the FDA, AMIC intends to seek classification and clearance as a class II medical device through a de novo application.
AMIC said it partnered in August with third-party radiopharmaceutical research and development firm IsoTherapeutics Group to provide commercialization support and conduct additional studies as requested by the FDA for Y-90 RadioGel. The project's first phase -- technology transfer, manufacturing, and in vitro testing -- has been completed. The next stage, which will involve additional in vitro and in vivo testing, began last Friday, according to the company.