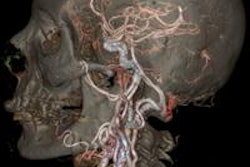
GE Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Q.Clear PET iterative reconstruction algorithm.
Designed as a tool to assist in diagnosis, staging, treatment planning, and treatment assessment, Q.Clear can improve both quantitative accuracy and image quality in PET/CT imaging, according to the vendor.