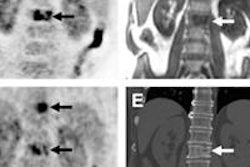
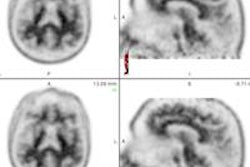
Radiopharmaceutical firm Navidea Biopharmaceuticals said the U.S. Food and Drug Administration (FDA) has accepted its supplemental new drug application (sNDA) and granted priority review for an expanded use of its Lymphoseek imaging agent.
Navidea is seeking additional clearance for Lymphoseek for the detection of sentinel lymph nodes in patients with head and neck cancer. The agent is currently approved for use in lymphatic mapping procedures performed to aid the diagnostic evaluation of lymph nodes draining a primary tumor in patients with breast cancer and melanoma.
The FDA has set June 16 as a target review date for the sNDA, Navidea said.