Siemens Healthcare plans to launch its new Biograph mCT Flow PET/CT scanner this weekend at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Vancouver, British Columbia.
Among its features, Biograph mCT Flow offers FlowMotion technology, which is designed to customize the imaging of organs into as many as four zones per scan.
"Up to this point, everyone in PET has designed the scans around the system, and the challenge has been making the anatomy fit into the field-of-view in the scanner system," James Williams, PhD, CEO of Siemens' Molecular Imaging unit, told AuntMinnie.com. "We are adapting the scanner to the anatomy of the patient. We can choose how much sensitivity and resolution we want to apply to anatomical regions, instead of bed-based, fixed-size windows."
For example, zone 1 may be designated as the head and neck region, zone 2 may scan the lungs at a slower pace for patients suspected of having lung cancer, zone 3 might include the liver, and zone 4 could be a faster scanning speed for lower regions such as the extremities.
"The big difference is that we can maximize the efficiency of the protocol by maybe spending more time, for instance, in the head and neck to get higher sensitivity and better image quality, or use a respiratory-gated protocol in the thorax," Williams explained. "It would allow you to adapt that to different patient sizes as well, so you get the same consistent image quality for the designated anatomical region."
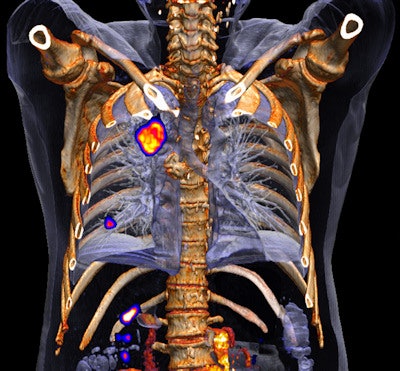 Biograph mCT Flow is designed for routine use of HD-Chest motion management techniques that enable delineation and quantification of small lesions. Image courtesy of University of Tennessee, Knoxville.
Biograph mCT Flow is designed for routine use of HD-Chest motion management techniques that enable delineation and quantification of small lesions. Image courtesy of University of Tennessee, Knoxville.Biograph mCT Flow also features a new table with Siemens' Molecular and Anatomical Registration Technologies (SMART) to enhance patient positioning from the CT exam at the front of the gantry through the PET scan at the back of the system.
The hybrid scanner has a 78-cm bore, which is large enough to handle bariatric patients, and a table capacity for patients who weigh as much as 500 lb. The table can also travel up to 200 mm per second when positioning a patient to improve workflow.
"The table is an important part of the whole FlowMotion system," Williams added. "We need very highly accurate calibrated movement of the table to achieve FlowMotion."
Patient scan time depends on the clinical application and to some extent on the scanner itself. Biograph mCT Flow can be configured with a three- or four-ring PET detector for enhanced image quality and reduced radiation exposure. Clinicians can reduce the dose of a radiopharmaceutical by as much as 50% and still acquire the same imaging data over the same length of time.
"The biggest benefit is being able to differentiate between how you want to scan the thorax, the head, the neck, and the lower abdomen," Williams said. "If we are doing any kind of scan for metastatic disease and looking for small metastases throughout the body, it allows us to get respiratory gating in the thorax area."
Biograph mCT Flow and FlowMotion technology have received 510(k) clearance from the U.S. Food and Drug Administration (FDA), as well as CE Mark in Europe. Siemens has already installed a Biograph mCT Flow system at the University of Michigan.
The company plans to begin shipping additional units within the next three months.