Radiopharmaceutical developer Navidea Biopharmaceuticals has entered into an agreement with Maimonides Medical Center in New York City to investigate the utility of its Lymphoseek product in lymphatic mapping procedures for colorectal cancer.
Dr. Danny Sherwinter, director of the department of minimally invasive and bariatric surgery at Maimonides, will lead the clinical study. The open-label evaluation, scheduled to begin in the first half of 2013, plans to enroll up to 40 colon cancer patients.
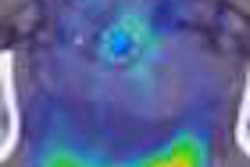
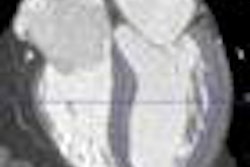
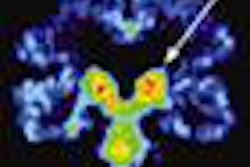
Lymposeek is an investigational radiopharmaceutical used in lymphatic mapping procedures to help stage cancers such as breast cancer and melanoma. It is designed to identify the lymph nodes that drain from a primary tumor.
The American Cancer Society estimates that approximately 140,000 new cases of colorectal cancer will be diagnosed in the U.S. in 2013. The worldwide annual number of newly diagnosed cases is estimated to be more than 1.2 million.