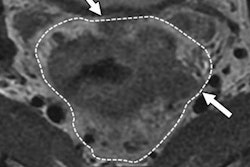
PET/CT results can predict which patients will respond to high-dose-rate endorectal brachytherapy for stage II and III rectal cancer, according to a study presented at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium. The results could pave the way for easier treatments and better outcomes.
Neoadjuvant chemoradiation followed by surgery is the standard of care for patients with rectal adenocarcinoma. But researchers at John Hopkins University are looking at the use of high-dose-rate endorectal brachytherapy (ENDO-HDR) in select patients to see if it might allow them to skip the chemotherapy, proceed to surgery after a few rounds of ENDO-HDR, and hopefully live out their lives in health.
First, though, they needed to demonstrate that PET could predict pathological response postsurgery in patients who received the short high-dose-rate radiotherapy protocol before surgery.
As hoped, the study showed that a reduction in peak standardized uptake value (SUVpeak) of more than 50% correlated with an absence or nearly complete absence of residual tumor cells after surgery, Dr. Rachit Kumar, a fourth-year resident in radiation oncology, told AuntMinnie.com.
"We're hoping to see if we can predict which patients don't need surgery, or whether some patients can have less surgery and fewer side effects and morbidity," Kumar said. "That would be a huge finding in this disease. ... The current process of getting chemo and radiation takes about five weeks, which is quite a long time, and we don't know the response rate that we're getting."
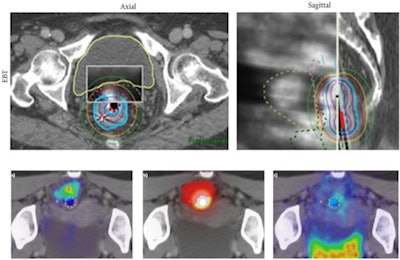 |
| Top row, ENDO-HDR planning CT and MRI with dose distribution. Bottom row, dose response as measured by F-18 FDG PET uptake. Lower left image, FDG fusing to tumor prior to brachytherapy; lower middle, ENDO-HDR dose distribution (bright spot) and tumor target volume (in red); lower right, post-ENDO-HDR therapy PET scan showing tumor response to therapy. Images courtesy of Dr. Rachit Kumar. |
The five-year survival of patients with stage II or III rectal cancer ranges from 50% to 70%, though the 50% number is somewhat optimistic for patients who have locally very advanced nonmetastatic disease, Kumar said.
High-dose localized radiation without chemotherapy before surgery has already been successfully applied by a Canadian team -- Vuong et al at McGill University -- whose five-year data look very promising, he said.
"We felt it was something we wanted to try here in the U.S.," Kumar said. "It would save time, it would avoid the early side effects of chemotherapy, and this high dose of radiation could kill the tumor a bit better."
As CT and MR alone aren't capable of predicting pathologic response in this setting, Kumar, along with Dr. Avani Dholakia, Dr. Joseph Herman, and colleagues, investigated the use of PET response (PERCIST) to predict pathological change in rectal cancer treated with ENDO-HDR.
The small prospective trial has analyzed the treatment results of nine patients to date, with many more to come, Kumar said. For the ENDO-HDR treatment, patients received 6 Gy per fraction for four fractions (total dose 26 Gy) in a neoadjuvant setting followed by surgery for stage II or III rectal cancer. ENDO-HDR is administered through a probe inserted into the patient.
During treatment with ENDO-HDR (Nucletron), "everyone leaves the room except for the patient, and we turn on the machine, which has a very highly active radioactive source within it that travels through a hollow tube into the applicator, which is in the patient," Kumar explained. Once the treatment is finished, the source that was in the hollow tube goes back into the machine that holds it, and other people can return to the room. The advantage of the technique is that the patient leaves the treatment without a radiation source, Kumar added.
The study team compared preradiation PET/CT to postradiation PET/CT a month following completion of radiation. PERCIST criteria were used to quantify tumor response in SUVpeak. Finally, surgical specimens were analyzed for degree of pathologic response using tumor response grade (TRG) per Mandard et al.
Patient data after ENDO-HDR treatment
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| PMR = partial metabolic response (SUVpeak decrease of 30% to 99%); S = stable metabolic disease (SUVpeak between 0 and 29%); pCR = pathologic complete response |
As shown in the table above, three of five patients who achieved TRG 1 or TRG 2 had an SUVpeak that decreased by at least 50% following therapy. Moreover, none of the four patients who did not achieve TRG 1 or TRG 2 had an SUVpeak change of 50% or more, the authors noted in their ASCO poster.
Despite the positive trend, the small sample size limits statistical significance (p = 0.17), Kumar said. Nevertheless, a statistical comparison of patients whose SUV decreased by 50% or more correlated with TRG 1 or TRG 2 demonstrated a positive predictive value of 100%, negative predictive value of 67%, specificity of 100%, and sensitivity of 60%.
"Six of the nine patients had a partial metabolic response, where their SUVpeak went down by at least 30%," he said. Previous studies have used SUVmax, an inferior measure of response in Kumar's view. The group is hopeful that the technique will allow for the nonoperative management of many patients.
Kumar acknowledged that the lymph nodes are not effectively treated when radiotherapy is applied endorectally. However, in patients treated so far, the rate of lymph node involvement later on has not exceeded that of conventional radiotherapy, he said.
"We feel like this is a very exciting early study," he said. "We're seeing something in these nine patients that we were hoping to see: that a decrease in the [SUVpeak] can help us predict which patients are going to respond. We still have a ways to go in the number of patients so that we can definitively say this, and we'll be working with the group in Canada to help analyze their data as well."
The researchers are still actively recruiting patients and hope to have the results of 40 or 50 for a more complete analysis before publishing the first peer-reviewed study, Kumar said. He cautioned that the technique continues to be applied very selectively.