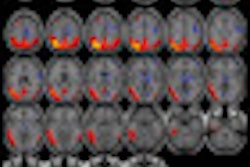
Radiopharmaceutical developer Navidea Biopharmaceuticals said that its partner AstraZeneca has completed a clinical trial of its NAV4694 radiopharmaceutical as a biomarker for visual detection and quantification of cerebral beta amyloid in diagnosing Alzheimer's disease.
The study was designed to assess the effects of various mass amounts of AZD6494 (NAV4694) on the safety and efficacy of PET scanning in subjects with Alzheimer's and in healthy volunteers. The open-label, nonrandomized, multicenter study included 16 individuals who were each imaged on two PET systems at Karolinska Institute sites in Stockholm.
Results from the study are expected to be presented at this year's Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting. A phase III study is planned for this year.
In other news, Navidea said it has obtained rights to two additional beta-amyloid imaging agents from AstraZeneca. The agents, AZD2184 and AZD2995, are carbon-11 (C-11) PET radioligand tracers discovered by AstraZeneca in collaboration with Karolinska Institute, according to the firms.
Both Navidea and AstraZeneca believe that the agents may serve as useful laboratory tools to support additional investigation of Alzheimer's. Financial terms of this deal were not disclosed, Navidea said.