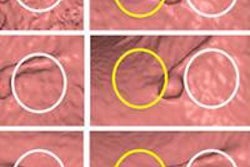
Virtual colonoscopy researchers have served up all the evidence the U.S. government asked for, and as a result they've concluded the time is now for approval of the technique to screen for colorectal cancer, according to an article published in the July American Journal of Roentgenology.
Four years have passed since the U.S. Centers for Medicare and Medicaid Services (CMS) nipped virtual colonoscopy screening (also known as CT colonography or CTC) in the bud by branding it with an "insufficient evidence" (I) rating by the U.S. Preventive Services Task Force (USPSTF).
Since then, a wide range of studies have addressed the three major concerns expressed by CMS:
- Radiation exposure
- Applicability of screening generally recommended for individuals 50 and older to the older population served by Medicare
- What to do about extracolonic findings, if follow-up could lead to additional tests, risks, and expenses
Those questions have been satisfactorily answered, and USPSTF's "I" rating is no longer valid, according to the authors of the AJR article, Dr. Judy Yee, Dr. Kathryn Keysor, and Dr. David Kim.
"The abundance of new research published since then in the peer-reviewed literature addressing the concerns of these organizations clearly document that the time has arrived to approve national reimbursement of CTC for screening of the general population," they wrote (AJR, July 2013, Vol. 201:1, pp. 73-79).
Is now really the time?
But is now really the time, considering how challenging it has been in recent years to get reimbursement for new screening procedures?
"Back in 2008 and 2009, we were at the start of a recession, and there was increased public attention to radiation dose that contributed to some of the delay in national coverage by CMS," Yee said in an interview with AuntMinnie.com. "And the issues that officially came out from Medicare -- the three main issues of concern -- have been dealt with."
Yee is professor and vice chair of radiology and biomedical imaging at the University of California, San Francisco, and she is chief of radiology at the San Francisco Veterans Affairs Medical Center.
Radiation
CTC using modern techniques exposes patients to 3 mSv or less of radiation -- on par with annual background radiation exposures in the U.S. With the newest techniques such as iterative reconstruction, exams can be provided at less than 1 mSv, the authors noted. At these low levels, the impact of radiation exposure is either too small to be demonstrated or does not exist, they wrote, echoing the conclusions of the Biological Effects of Ionizing Radiation (BEIR VII) panel.
Several other organizations have agreed with those conclusions, including the National Health Physics Society in 2010 and the American Association of Physicists in Medicine (AAPM) in 2011. AAPM discourages calculation of any radiation risks below 50 mSv because the risk is too small to estimate.
"Current methods are widely available to achieve low radiation dose for CTC including decreasing tube current or tube voltage as well as the use of automatic tube current modulation and iterative reconstruction technique," Yee and colleagues wrote. "Many sites have decreased CT radiation dose by instituting tube current reduction, which has already been previously shown to be a useful strategy for CTC."
Among the research cited, a 2011 study by Berrington de González and colleagues calculated the risk-benefit ratio for CTC versus the estimated radiation risk. They found a "large benefit-to-risk ratio in favor of screening CTC that varied from 24:1 to 35:1," the authors wrote.
Extracolonic findings
Since 2008, 10 research studies have been published on extracolonic findings, Yee and colleagues noted. The studies help predict the potential costs related to extracolonic findings at CTC if screening were implemented.
In a 2012 study, Zalis and colleagues identified indeterminate findings (rated E3 by the C-RADS lexicon) in 16% of participants and potentially significant findings (E4) in 3%. However, only 5% of these findings actually underwent evaluation, seriously reducing the cost and potential harms related to follow-up. Even when they are followed, most investigations are limited to a second imaging exam.
"Thus, the use of other measures such as the overall extracolonic finding rate, reported to range from 60% to 70%, or extracolonic findings of potential clinical significance, reported to range from 10% to 16%, as a proxy for CTC burden grossly overestimates the true percentage," Yee and colleagues wrote. "When an electronic medical review is undertaken to determine what actually happened, additional diagnostic examinations for incidental extracolonic findings occur typically in fewer than 10% of screened patients. This has been consistently observed throughout the CTC literature."
Similarly, studies of more than 2,000 screening participants reported that of the 9.3% of the potential pool of patients with E3 or E4 findings, only 6.1% underwent workup.
"And, finally, it is important to remember that real benefits arise from incidental extracolonic findings in the diagnosis of unsuspected cancers and vascular aneurysms," the authors wrote.
Medicare-aged population
CMS concluded in May 2009 that evidence did not support expansion of CTC to Medicare beneficiaries, writing that current studies did not show a generalizability of existing data to these beneficiaries, who are typically 15 to 25 years older than the recommended starting age of 50 for colorectal cancer screening.
There has "not historically ever been a requirement for studies specific to the Medicare-age population," but investigators have produced data anyway. Sizeable studies based on National CT Colonography Trial (ACRIN 6664) data, as well from the National Naval Medical Center, the University of Wisconsin, and other centers, have found very similar, if slightly higher, rates of significant colonic and extracolonic findings in older patients.
In 2010, for example, a study by Kim and colleagues found "no statistically significant difference in the major characteristics of advanced neoplasms including size, histology, morphology, and location when comparing the older cohort with the general screening cohort."
The prevalence of advanced neoplasia was 7.6% for the older age group, double the rate for younger patients, but acceptable from a screening view, since most patients would still not require referral for colonoscopy. Based on a 6-mm threshold used to indicate a positive finding, the overall senior patient referral rate was 15% for colonoscopy. When only adenomas were considered, per-patient positivity rates for 6-mm and 10-mm thresholds were 11% and 7%, respectively.
Next steps
USPSTF is currently scheduled to review colorectal cancer screening this fall, and if the recommendation is positive, CTC can potentially proceed automatically to screening coverage without the hurdle of congressional action, Yee said.
"It's formally on the [USPSTF] agenda this fall -- not just CTC but all colorectal cancer screening," she said. "It's a long process that takes about 18 months; however, the two agencies, CMS and USPSTF, have agreed to work together so that if there is a positive outcome, it will be a quicker route for the final CMS approval."
As the AJR article notes, the Patient Protection and Affordable Care Act (PPACA) of 2010 (aka Obamacare) requires insurers to provide free coverage, without patient cost sharing, for all preventive services that receive an "A" or "B" rating from USPSTF, signifying the highest levels of efficacy. Thus, should CTC be granted a favorable rating from the task force, progress toward national screening coverage would likely be fast. In fact, USPSTF and CMS have already signaled that the new evidence may be sufficient.
"We actually met with CMS and USPSTF and asked each member of the group whether they saw any additional evidence gaps, and the response was no," Yee said.
The many new peer-reviewed studies since 2008 "clearly document that the time has arrived to approve national reimbursement of CTC for screening of the general population," the authors concluded. CTC has already been endorsed by the American Cancer Society, the U.S. Multisociety Task Force, and the American College of Radiology as a reliable test for colorectal cancer screening, they added.
"National reimbursement will allow more rapid and widespread dissemination of CTC to the public, which will result in improved [colorectal cancer] screening adherence and ultimately in more lives saved," they wrote.
For her part, Yee seems increasingly confident that this time, national coverage has a very good chance of approval.
"I am very hopeful," she said. "I think that we have stepped up to the plate in pretty short order to respond to concerns that CMS and USPSTF have had. In addition, there's been a technological evolution since 2008 and 2009 that's allowed us to significantly reduce dose."