Toshiba America Medical Systems has received U.S. Food and Drug Administration (FDA) clearance for its Aquilion Prime CT scanner.
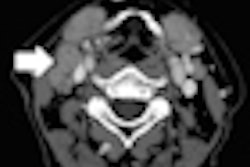
Prime features double-slice technology and Toshiba's coneXact reconstruction algorithm, allowing for generation of 160 unique slices per rotation, according to the vendor. The scanner is targeted for facilities that need to perform a wide variety of advanced clinical exams and produce high-quality images with reduced radiation exposure.
Prime includes an 80-row, 0.5-mm detector; a 7.5-million-heat-unit (MHU) large-capacity x-ray tube; and 0.35-sec gantry rotation speed, Toshiba said. It also has a 78-cm aperture gantry and a couch with a patient-weight capacity of 660 lb.
The company's adaptive iterative dose reduction (AIDR) and NEMA XR 25 dose-check software are standard features on Prime.