In little more than a decade, imaging and treating the acute stroke patient has progressed from science experiment to lifesaver. Patients fortunate enough to be treated quickly are spared critical brain function that would have been lost in an era before recombinant tissue plasminogen activator (rtPA) was available for patients selected with advanced imaging techniques such as diffusion-weighted MRI and CT perfusion.
But much is left to accomplish, as stroke imaging expert Dr. Max Wintermark said in a recent presentation. Researchers aim to create more accurate representations of salvageable brain tissue, using CT, MRI, and nuclear medicine. Each modality visualizes infarcted brain tissue differently; even within modalities, results can vary widely based on different threshold settings, processing techniques, blood vessels, and even different observers.
Our two-part series on acute stroke imaging examines controversies and discoveries in imaging and treating ischemic stroke patients, based on presentations at the 2007 RSNA meeting in Chicago. In part II, Dr. Max Wintermark examines the range of imaging representations for infarcted brain tissue, with an eye toward future research and collaboration.
The penumbra model
By now most radiologists are familiar with the classic penumbra model in acute ischemic stroke imaging: An infarct core consisting of completely dead brain tissue lies at the center, surrounded by tissue that is still alive but at risk, then a region of benign oligemia that is impacted but not at risk, and finally normal brain parenchyma.
"When you try to translate this physiological concept to the imaging modality you are using, you have to choose every day (at) which level you want to translate it," Wintermark said.
"You can use a vascular definition based on blood volumes, based on cerebral blood flow and mean transit time through the vascular territory," Wintermark said. "You can use a metabolic definition ... with sodium imaging, or you can use a molecular definition to try to characterize brain ischemia."
Wintermark, who received his radiology training at Lausanne University Hospital in Switzerland, is currently in residence as an assistant professor of neuroradiology at the University of California, San Francisco.
"Several models have been proposed, but none of these models has been formally compared to each other in a large series, (has) not been validated," Wintermark said in his talk. "That's perhaps the reason why there's not one standard imaging technique used to image stroke."
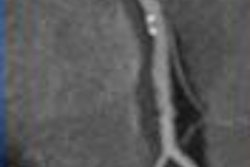
The original penumbra model relied on the mismatch between perfusion and diffusion on MRI -- the mismatched region representing the tissue you want to save, he said. In CT there is no diffusion, only perfusion, which is measured by mean transit time (MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV). The way to distinguish potentially salvageable tissue from the infarct core in CT is by via cerebral blood volume and mean transit time through the cerebral vasculature, he said.
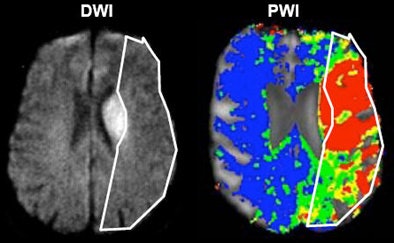 |
| Acute ischemic stroke visualized on diffusion-weighted MRI (left) and perfusion CT (right). All images courtesy of Dr. Max Wintermark. |
Autoregulation
In ischemic stroke, a reflex known as autoregulation initially compensates for reduced blood flow through vasodilation of resistance arterioles. If the occlusion is of short duration, cerebral blood volume will be maintained; if not, it will eventually fall, Wintermark said. This reflex is not accounted for in the classic penumbra model.
But it is accounted for in a model proposed by Canadian researchers Ting Lee and colleagues, who aimed to determine whether CT perfusion-derived CBF and CBV could be used to differentiate between penumbra and infarcted gray matter in 30 acute stroke patients.
The patients underwent noncontrast CT angiography and perfusion CT scans in the first week after acute stroke, and were divided into patients who were recanalized and nonrecanalized during the first 24 hours.
"For recanalized patients, CBF was significantly lower (p < 0.05) for infarct (13.3 ± -3.75 mL x 100 g-1 x min-1) than penumbra (25.0 ± -3.82 mL x 100 g-1 x min-1)," Lee's team wrote in Stroke. "CBV in the penumbra (2.15 ± -0.43 mL x 100 g-1) was significantly higher than contralateral (1.78 ± -0.30 mL x 100 g-1) and infarcted tissue (1.12 ± -0.37 mL x 100 g-1). Logistic regression analysis based on CBF and CBV interaction yielded sensitivity, specificity, and accuracy of 97.0%, 97.2%, and 97.1%, respectively, for determining which patients had been recanalized" (Stroke, July 2006, Vol. 37:7, pp. 1771-1777).
CBF and CBV can be both sensitive and specific for infarction, and should be studied further for its potential in differentiating infarct from penumbra, concluded the team from the University of Western Ontario in London, Ontario, Canada.
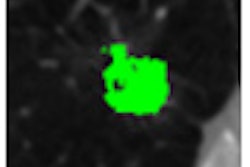
Ting's team "used the concept of autoregulation, and different combinations of CBF and CBV to try to distinguish between the infarct core and tissue at risk, so this is a slightly more complex model than what was used originally for diffusion-perfusion MRI," Wintermark said. All the current CT penumbra models, including his own, incorporate autoregulation, Wintermark said.
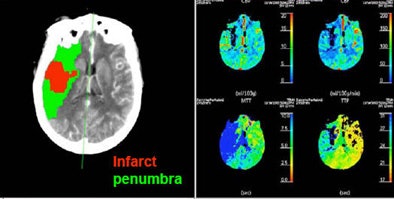 |
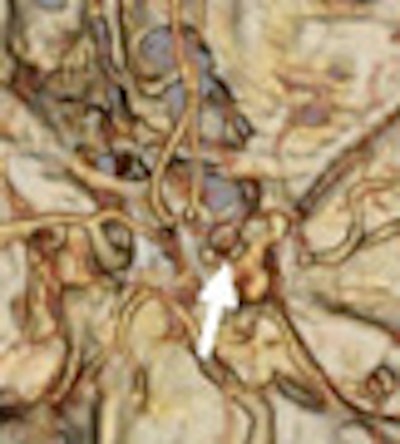
| Patient with middle cerebral artery stroke underwent perfusion CT. Penumbra model (Wintermark et al) demonstrates infarct in red and penumbra in green, incorporating the concept of autoregulation. |
Other penumbra models
Wintermark cited several complex penumbra models that have been proposed to improve the delineation of potentially salvageable tissue from the infarct core. Among them, a model by Dr. Ona Wu and colleagues from the University Medical Center Utrecht in Utrecht, Netherlands, and Massachusetts General Hospital in Boston employed a voxel-based generalized linear model algorithm to predict tissue infarction in animals, which permitted a comparison of predicted outcomes to histology.
The model was used to predict infarction on a voxel basis for either saline or rtPA treatment in 13 rats. The predicted outcome was 92% accurate in saline-treated rats, but significantly reduced (86%) in rtPA-treated animals (p = 0.04), Wu's team wrote (Journal of Cerebral Blood Flow & Metabolism, January 2007, Vol. 27:1, pp. 196-204).
"MRI-based algorithms can predict tissue infarction with high accuracy in animals not receiving thrombolytic therapy," the group concluded. "Furthermore, alterations in disease progression due to treatment were more sensitively monitored with our voxel-based analysis techniques than with volumetric approaches."
This study used not only diffusion and a single perfusion map, but different MRI sequences to create the generalized linear model, Wintermark said.
Dr. Chelsea Kidwell and colleagues from the stroke center at the University of California, Los Angeles, are using diffusion-weighted MRI to predict which rtPA-treated stroke patients will go on to suffer hemorrhagic transformation, Wintermark said.
There are also studies on the use of sodium concentration as a measure of tissue viability after stroke, such as the study initially reported by Thulborn et al (Radiology, October 1999, Vol. 213:1, pp. 156-166). Sodium is slow, however, requiring 30 to 40 minutes, Wintermark said.
Perfusion models and parameters
Just as there are many models of penumbra, there are many different models of perfusion, from dynamic perfusion CT to dynamic susceptibility contrast T2-weighted MRI -- to models that use deconvolution versus no convolution, and qualitative versus quantitative slopes.
And because software algorithms based on these models use different parameters (such as mean transit time, time to peak), output can vary widely. Depending on how the software manufacturer designs the algorithm, variations can occur even within the same patient data.
"Typically, when you talk about mean transit time, you have to remember that MTT doesn't always mean the same thing," Wintermark said. "Does the software use a deconvolution approach? You have to very careful. Even if you are using mean transit time and cerebral blood volume to predict your infarct core and your penumbra, not all software will give you the same CBF and CBV values."
Processing affects output
Take care to use a single penumbra model and a single perfusion model -- and a certain amount of interobserver variability will still occur, Wintermark said. Different processing methods also affect the output.
In perfusion CT imaging of acute stroke, for example, arterial selection influences mean transit time and regional cerebral blood flow, but does not affect regional cerebral blood volume.
The vessel in which perfusion is measured also affects output; for example, the underlying imaging method can use arterial input or venous reference.
"The arterial input function is used as a reference to calculate MTT, while the venous reference is used to calculate cerebral blood volume," Wintermark said. "Depending on which artery you pick, it can influence ... mean transit time. In acute stroke, it doesn't matter much, but when you have a chronic vascular condition (such as carotid artery stenosis), it can make a big difference."
As for the venous reference, choosing a vein at a lower level works well for anatomical reasons, he said. But an upper-level vein, if not selected properly, can lead to an overestimation of CBV values.
"If you select (the artery) inappropriately, you are going to get an incorrect MTT map," Wintermark said. "Similarly, if you don't select the appropriate vein, you are going to get an incorrect CBV map. Then, if your MTT and CBV maps are not correct but you apply some thresholds that are correct, you are going to get inaccurate info about the infarct and penumbra."
In any case, it's best to use a single vein for all your boluses and all of your slices, he said.
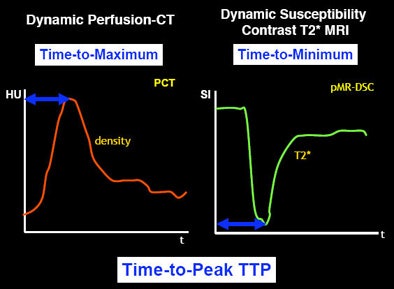 |
| Different parameters used in different imaging modalities (or within a single modality) can alter the input into the software and hence the perfusion map for stroke patients. |
 |
MR and CT
The modality makes a difference in the results, too, but CT and MRI have more commonalities than differences, Wintermark said, adding that each has its specific advantages and disadvantages. Unlike MRI, CT cannot diagnose tissue viability, only tissue perfusion, he said.
"If you are expecting a very small stroke, you are probably not going to see it on the perfusion CT because the spatial resolution will be insufficient -- but you are going to see it very clearly on MR diffusion," he said. Also, due to a lack of spatial resolution, perfusion CT is usually inferior to MR for the posterior fossa. CT can miss lacunar infarcts, he said.
If you're trying to protect the orbits from radiation during a CT scan, you're not going to see the brain stem, he said.
Thinking ahead
On the other hand, CT recently improved substantially with the development of 256-slice (now 320-slice) CT (AquilionONE, Toshiba America Medical Systems, Tustin, CA). With CT's coverage as broad as that of MRI, if CT misses a stroke, it won't be for lack of appropriate coverage, Wintermark said.
In the years ahead, look for important developments in delay-insensitive deconvolution processing, which Wintermark called his personal favorite method. Automated arterial input and venous output functions will eliminate the uncertainties associated with picking the best artery and vein because it will be handled automatically.
The near future will also bring validation of the various methods in larger patient cohorts, Wintermark said. Reperfusion and collateral vessels will take on new importance.
Large studies will have to look at outcomes to determine whether a treatment is effective or not. "To reopen an artery that was closed does not mean the treatment was completely efficient," he said. The next few years will bring new neuroprotective agents and new reperfusion therapies as well.
"There are some challenges, but people know what those challenges are, so it's just a matter of working together to fix them, and we are working together," he told AuntMinnie.com.
Addressing attendees of the International Stroke Conference 2008 in New Orleans in mid-February, Wintermark said he discussed a new collaboration with stroke imagers worldwide, known as STIR (stroke imaging repository), that aims to conquer all of the outstanding issues.
When pharmaceutical companies get the message that stroke imaging is difficult and the methods not yet validated, "they think about giving up on stroke, and I think that's the wrong message," Wintermark said. "When everyone is working together on issues, that can change dramatically the way it goes in the future."
By Eric Barnes
AuntMinnie.com staff writer
March 3, 2008
Related Reading
tPA treatment window can be widened in some ischemic strokes, February 25, 2008
Part I: Stroke imaging hones in on preventable infarction, February 21, 2008
SWI MR reveals microarchitecture of neurovascular diseases, February 1, 2008
New indicators predict outcomes of tPA treatment for stroke, August 14, 2007
Diffusion-weighted MRI: The cornerstone of neuroimaging-based stroke studies, June 14, 2007
Copyright © 2008 AuntMinnie.com