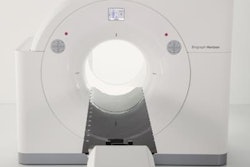
The U.S. Food and Drug Administration (FDA) has cleared Siemens Healthineers' Somatom go CT scanner line.
The 32-slice Somatom go.Now and 64-slice Somatom go.Up CT scanners offer automated, standardized workflows using a tablet computer. Users can control routine examinations using only the tablet remotely; standardized work steps allow users to run the scan with a few inputs. A new Stellar detector has integrated electronics and spectral tin filters.
All computer hardware formerly located in the control room is now integrated into the gantry, so Somatom go systems can be installed in one room with a minimum size requirement.
To develop the system, Siemens held interviews and workshops with more than 500 radiologists, radiologic technologists, and radiology administrators worldwide.