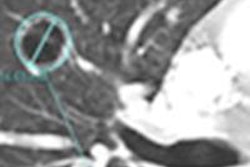
Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its planning application for transcatheter aortic valve implantation (TAVI) treatments.
Through 3D imaging, Philips' TAVI application offers interventionalists tools for preprocedural positioning to treat aortic stenosis. The planning application is available as part of Philips' IntelliSpace Portal 6 advanced visualization package, according to the firm.