Cardiac image analysis software developer HeartFlow has received U.S. Food and Drug Administration (FDA) clearance for its FFR-CT software, which noninvasively estimates fractional flow reserve (FFR) from cardiac CT studies.
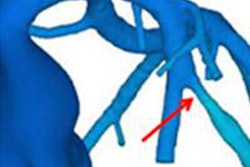
HeartFlow FFR-CT is a computer modeling program that assesses blood flow in the coronary arteries of patients with signs and symptoms of coronary artery disease, according to the FDA. Healthcare professionals can send a patient's CT scan data to HeartFlow, where a case analyst creates 3D computer models of different sections of the heart and runs a blood flow simulator program on the models.
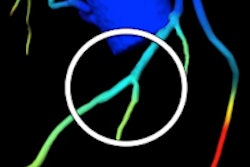
After analyzing the data and the models, the case analyst electronically sends a report with the FFR-CT values displayed as color images of the patient's heart. This estimate, along with other clinical patient data, can be used by healthcare professionals to determine the likelihood that the actual FFR is below accepted limits and whether or not a more accurate FFR assessment using cardiac catheterization is needed, according to the FDA.