Hologic of Bedford, MA, has received U.S. Food and Drug Administration clearance to market the use of its Discovery dual-energy x-ray absorptiometry (DEXA) system for cardiovascular applications.
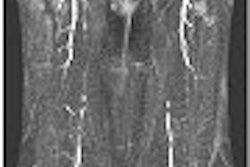
The clearance covers the use of Discovery for visualization of abdominal aortic calcification (AAC), which can be a predictor of coronary heart disease, stroke, and other forms of cardiovascular disease. Discovery users can take data collected from a low-dose, 10-second instant vertebral assessment (IVA) osteoporosis test on Discovery to visualize the presence of AAC, the company said.
By AuntMinnie.com staff writers
May 5, 2006
Related Reading
Hologic buys FFDM supplier, May 3, 2006
Hologic books record Q2, April 25, 2006
Hologic to buy Suros, April 18, 2006
AIE, Hologic highlight research, March 29, 2006
Hologic, Johns Hopkins APL ink pact, February 7, 2006
Copyright © 2006 AuntMinnie.com