The U.S. Food and Drug Administration (FDA) has issued an advisory that warns mammography providers that images from some full-field digital mammography (FFDM) systems may not be displaying properly on PACS workstations.
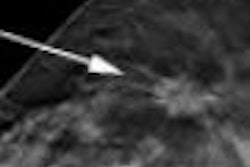
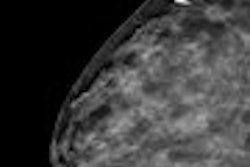
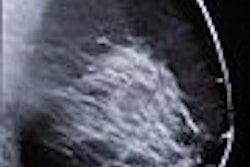
In an advisory sent on July 29, the FDA said that when images from some FFDM units are displayed on a workstation from a third-party PACS vendor, "the image identification information may obscure breast tissue on hard-copy images ... or the view and laterality may not always appear near the axillary portion of the breast in either the soft- or hard-copy images," in violation of rules in the Mammography Quality Standards Act (MQSA).
The flaw may result in pathology being overlooked or incorrectly localized, the advisory said. The situation is especially important if images are being sent to another facility that is unfamiliar with the configuration of equipment used to acquire the mammogram.
The FDA is advising all facilities using FFDM systems to check that image-identifying information is correctly displayed on soft- and hard-copy images.
Related Reading
FDA says mammo center defaults on MQSA fine, July 8, 2008
FDA proposes easier regulatory path for FFDM systems, June 10, 2008
Copyright © 2008 AuntMinnie.com