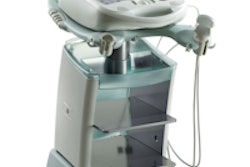
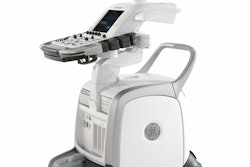
GE Healthcare and partner Deep Breeze have received U.S. Food and Drug Administration (FDA) 510(k) marketing clearance for their VRIxv lung imaging system.
The vibration response imaging (VRI) system is a noninvasive and radiation-free method that records lung sounds by utilizing acoustic sensors brought in contact with the patient's back while lying in bed, according to GE of Chalfont St. Giles, U.K., and Deep Breeze of Or-Akiva, Israel. Software then converts these signals into dynamic lung images with regional distribution maps, numerical representation, and breathing graphs.
Related Reading
GE, Orbotech dispute goes to arbitration, December 9, 2008
GE partners with MERS, December 9, 2009
Report: GE plans cuts in 2009, December 5, 2009
GE launches PET/CT, x-ray, product branding at RSNA show, December 2, 2008
FDA clears Deep Breeze lung imaging unit, August 9, 2007
Copyright © 2008 AuntMinnie.com