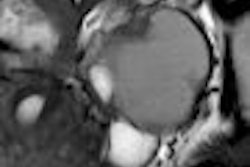
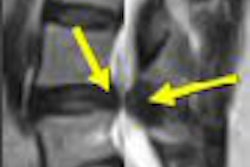
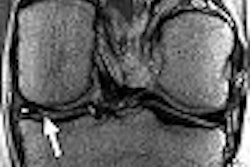
Nearly a quarter of U.S. women suffer from clinically symptomatic uterine fibroids. While treatment options have moved beyond hysterectomy, researchers from New York City asked how many of these women are eligible for a less invasive treatment, such as MR-guided focused ultrasound (MRgFUS). A fair number, according to the group, but refining MRgFUS will open it up to a broader patient population.
"(MRgFUS) is attractive given that it is completely noninvasive and requires little sedation and virtually no recovery," wrote Dr. Elizabeth Kagan Arleo and colleagues from New York-Presbyterian Hospital and Weill Medical College of Cornell University. "The recognized problems with the technique include its cost, the fact that viable fibroid tissue persists after most treatments that could serve as a nidus for regrowth, and the fraction of patients with symptomatic fibroids that are eligible for MRgFUS" (Journal of Vascular and Interventional Radiology, May 2007, Vol. 18:5, pp.681-685).
For this retrospective review, the researchers included women who had inquired about minimally invasive treatment options for uterine fibroids. Of course, a desire to learn more about less invasive procedures wasn't enough. The patients had to meet certain criteria. First, they underwent screening with contrast-enhanced and noncontrast pelvic MRI. Uterine fibroids had to be visible on noncontrast MR.
Next, the women had to be able to communicate sensations felt during the MRgFUS procedure, have a symptom severity score of 21 or more on the Uterine Fibroid Symptom and Health Related Quality of Life questionnaire. They also had to have kept all follow-up appointments.
Patients were excluded from the review if they had too many fibroids or too much fibroid volume because of U.S. Food and Drug Administration (FDA) limitations on maximum treatment time (three hours) and treatment volume (50% of fibroid volume), the authors explained. They then categorized the reasons the patients were, or were not, eligible for MRgFUS.
According to the results, out of 333 women, 37% were ineligible based on the inclusion and exclusion criteria. The top two reasons were insufficiently symptomatic fibroids and age (younger than 40 years or older than 60 years).
Anatomic reasons for ineligibility included abdominal scarring directly in the region where the FUS treatment beam would pass. "Scars in the beam path can become heated even by nonfocused acoustic energy and can lead to skin burns," the authors explained. A clinical call of ineligibility was made when there was too much fibroid volume.
In the end, 63% of the patients interested in MRgFUS were clinically eligible and 25% were also anatomically eligible. "Overall, 14% of women inquiring about minimally invasive image-guided treatments for fibroids were eligible for MRgFUS," the authors wrote.
While this percentage is respectable, they pointed out that improvement in MRgFUS technique could make it more feasible for a larger patient population. Some possibilities are pretreatment shrinkage of the fibroids with gonadotropin-releasing hormone agonists (GnRH-a) and the use of sonications with controlled microbubble formation.
MRgFUS still needs to gain greater acceptance in the clinical setting, they added. The results of a study presented at the 2007 American College of Obstetricians and Gynecologists (ACOG) meeting in San Diego advanced the MRgFUS cause. In a poster presentation, Dr. Phyllis Gee from the North Texas Uterine Fibroid Institute in Plano investigated the efficacy and durability of MRgFUS for the treatment of uterine myoma.
Gee found that patients treated with MRgFUS sustained a 10-point improvement in symptoms, as well as sustained symptom relief up to 36 months. In a second, related poster David Lee, Ph.D., determined that nearly 7% of women who underwent surgical treatment for uterine fibroids had to be retreated within one year. Lee is the senior director of Health Economics and Outcomes Research at GE Healthcare of Chalfont St. Giles, U.K. GE, along with InSightec of Haifa, Israel, developed the ExAlbate 2000 system.
By Shalmali Pal
AuntMinnie.com staff writer
June 18, 2007
Related Reading
MRI-guided ultrasound surgery improves fibroid symptoms, June 1, 2007
MR signal, nonperfusion drive long-term success of MR-guided focused US therapy, May 23, 2007
FDA clears InSightec's ExAblate 2000 for GE 3T MRI, March 1, 2007
Hormone therapy boosts efficacy of ultrasound therapy for uterine leiomyomata, July 5, 2006
Copyright © 2007 AuntMinnie.com