MRI contrast developer Epix Pharmaceuticals of Lexington, MA, said it will submit a formal appeal to the director of the Center for Drug Evaluation and Research (CDER) at the U.S. Food and Drug Administration (FDA).
Epix will ask CDER to overrule the decision by the Office of New Drugs (OND) that denied a previously submitted appeal for the company's Vasovist blood-pool imaging agent.
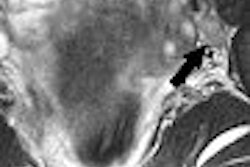
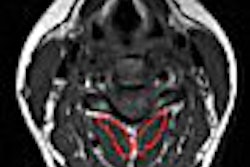
Vasovist is an injectable intravascular contrast agent designed to provide visual imaging of the vascular system through MR angiography. The FDA in 2005 issued two approvable letters for Vasovist, but made final approval contingent on the completion of at least one additional clinical trial for the product. Epix has been appealing that decision.
The company's newest appeal is in response to OND's denial in August this year of Epix's appeal to OND of the two approvable letters. Regulatory authorities in the European Community, Switzerland, Canada, and Australia have approved Vasovist for the visualization of peripheral vascular disease.
By AuntMinnie.com staff writers
December 15, 2006
Related Reading
Epix releases clinical trial results for blood-clot agent, November 30, 2006
Epix nets Canadian OK for Vasovist, November 6, 2006
Epix nets Australian approval, September 20, 2006
Epix regains Nasdaq symbol, September 15, 2006
FDA rejects Epix appeal, August 28, 2006
Copyright © 2006 AuntMinnie.com