The U.S. Centers for Medicare and Medicaid Services (CMS) has begun a regulatory process that could lead to reimbursement for PET bone scans using the fluorine-18 (F-18) sodium fluoride radiopharmaceutical. Nuclear medicine advocates believe that PET bone scans could provide an alternative to SPECT bone studies, which use the technetium-99m radiopharmaceutical that is currently in short supply.
CMS on June 4 announced that it was reconsidering its existing national coverage decision (NCD) not to cover sodium fluoride PET scans. The announcement opens a 30-day period for comments on the proposed coverage.
The agency's move to reconsider coverage is the result of a petition by the SNM of Reston, VA, and the Academy of Molecular Imaging (AMI) of Los Angeles asking for the change in policy, according to incoming SNM president Dr. Michael Graham, Ph.D., director of nuclear medicine at the University of Iowa in Iowa City.
Although CMS turned down a similar request earlier this year, the situation has changed due to the closure of the National Research Universal reactor in Chalk River, Ontario, which produces most of North America's supply of molybdenum-99 (Mo-99). Mo-99 decays into technetium-99m, which is the primary radioisotope used for SPECT bone studies.
The shutdown of regular Mo-99 shipments has forced many institutions like the University of Iowa to cut back drastically on the number of SPECT-based bone studies they're conducting, Graham said. The University of Iowa is getting by with technetium that's unused from facilities in other cities and has been forced to schedule bone scans on the weekends.
"Normally we get two technetium generators a week at our university due to our rural location, but we've received no generators now for two weeks," Graham said.
F-18 sodium fluoride PET could lighten some of the load because the radiopharmaceutical can be produced at the university's own cyclotron.
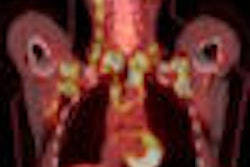
There are also clinical justifications for performing PET-based bone scans rather than SPECT. The image quality of PET studies is far superior to SPECT exams, and Graham estimates that even if Mo-99 were adequately supplied, PET would take over 10% to 20% of bone studies due to its better resolution.
"The image quality of sodium fluoride is quite a bit better than technetium-99m bone scans because of better imaging characteristics of PET," Graham said. "It makes absolutely stunning bone scans."
CMS earlier this year turned down a similar petition to revise its coverage position on F-18 sodium fluoride PET bone studies because of what it said was a lack of sufficient clinical trials showing efficacy. But ongoing problems with access to technetium-99m may force the agency's hand in another direction. Canada has already approved payments for PET bone studies, Graham said.
CMS is accepting comments on the proposed change through July 4, and the agency expects that it will make a decision by March 4, 2010. Information on the policy change can be reached by clicking here.
"If this were approved, we could make our own sodium fluoride, and the large suppliers would be able to provide it to their customers," Graham said. "It's not the perfect solution, but it is a stopgap solution."
By Brian Casey
AuntMinnie.com staff writer
June 5, 2009
Related Reading
Canada medical reactor shutdown may be extended, June 5, 2009
MDS advocates Maple reactor activation, June 1, 2009
AECL reactor down 'at least three months,' May 29, 2009
MDS Nordion opens new FDG facility in Belgium, May 28, 2009
AECL ponders Chalk River repairs, May 26, 2009
Copyright © 2009 AuntMinnie.com