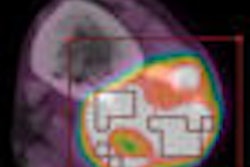
The U.S. Centers for Medicare and Medicaid Services (CMS) early Friday evening released its decision to change its policy so that all Medicare beneficiaries with certain cancers will be able to receive Medicare coverage for at least one PET scan, as prescribed by their physicians.
The CMS ruling also expands the coverage of the nine currently covered cancers to include the subsequent treatment strategy, in addition to initial diagnosis. The nine cancers are breast, cervical, colorectal, esophageal, head and neck, lymphoma, melanoma, non-small cell lung, and thyroid.
In addition, Medicare will expand coverage to include ovarian cancer and myeloma, making a total of 11 indications now covered for both the initial diagnosis and subsequent treatment strategy. For all other cancers, PET coverage for subsequent treatment strategy evaluation requires participation in an approved Coverage with Evidence Development (CED) program, such as a modified National Oncologic PET Registry (NOPR).
"This is a real step forward in adding ovarian cancer and myeloma to the list," said Dr. Michael Graham, SNM president-elect and director of nuclear medicine at the University of Iowa, speaking with AuntMinnie.com. "These are areas where there is good evidence that PET is very useful."
CMS' conclusions
With the decision, CMS concluded that the "evidence is adequate to determine that the results of FDG-PET imaging are useful in determining the appropriate initial treatment strategy for beneficiaries with suspected solid tumors and myeloma and improve health outcomes and thus are reasonable and necessary."
Therefore, CMS will "cover only one FDG-PET study for beneficiaries who have solid tumors that are biopsy-proven or strongly suspected based on other diagnostic testing when the beneficiary's treating physician determines that the FDG-PET study is needed to determine the location and/or extent of the tumor for the following therapeutic purposes related to the initial treatment strategy."
The decision replaces the four-part framework -- covering diagnosis, staging, restaging, and monitoring response to treatment -- with a two-part framework that differentiates the use of FDG-PET imaging in the initial treatment plan from other uses related to guiding subsequent treatment strategies.
In a written statement, Robert Atcher, Ph.D., president of SNM and professor of pharmacy at the University of New Mexico/Los Alamos National Laboratory, called the CMS decision "a major victory for patients. CMS' decision to cover PET scans for cancer demonstrates the intrinsic medical value of PET and important role of these scans in diagnosing, staging, restaging, and monitoring treatment for many cancers."
NOPR contributions
Today's decision comes just after the one-year anniversary of the initial request by representatives from NOPR for CMS to expand federal reimbursement for the use of FDG-PET in oncology applications. The March 25, 2008, communiqué asked CMS to expand FDG-PET coverage to the diagnosis, staging, and restaging of all oncology applications not covered by Medicare and Medicaid.
The regulatory process was established in May 2006, when the NOPR was created to collect data on PET's clinical value as a means of helping CMS determine whether to expand Medicare and Medicaid coverage. For 18 months, the NOPR collected data from more than 1,200 PET facilities across the U.S. to see how FDG-PET scans influence cancer treatment plans.
In its analysis of 22,975 patients, published online on March 24, 2008, in the Journal of Clinical Oncology, the study found that FDG-PET prompted changes in patient management in 36.5% of the cases. Within that 36.5%, FDG-PET results prompted a change from nontreatment to a treatment plan 28% of the time, while 8% of cases changed from treatment to nontreatment decisions.
Continuing research
Graham said the expectation is that NOPR will continue its work with cancers that remain unreimbursed by CMS. "There are other cancers where PET is almost certainly useful and that data must be obtained," he said. "That's why continuing NOPR is very important."
The NOPR is sponsored by the Academy of Molecular Imaging (AMI) of Los Angeles and is managed by the American College of Radiology (ACR) of Reston, VA, and the ACR Imaging Network (ACRIN).
To help PET providers better understand the new CMS policy, SNM will host a live audio conference on Monday, April 27, 1 p.m. to 2:30 p.m. Eastern time. For more information or to register for the audio conference, visit www.snm.org/PETChanges.
By Wayne Forrest
AuntMinnie.com staff writer
April 3, 2009
Related Reading
CMS proposes expansion of PET cancer coverage, January 7, 2009
NOPR: PET changes care of more than a third of cancer patients, October 6, 2008
CMS reconsiders PET coverage framework, September 16, 2008
NOPR asks CMS to expand FDG-PET coverage, April 11, 2008
NOPR study: PET influences cancer care, March 25, 2008
Copyright © 2009 AuntMinnie.com