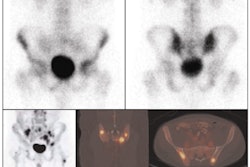
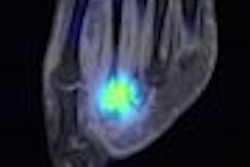
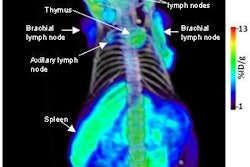
NeuroSurvival Technologies (NST) of Petach-Tikva, Israel, has received the go-ahead from the U.S. Food and Drug Administration (FDA) to begin clinical trials of ApoSense, a molecular imaging agent for programmed cell death.
ApoSense detects signs of apoptosis, a genetically controlled process of cell death that is associated with most medical disorders. Potential clinical applications include oncology, neurology, and cardiology.
Upon recognizing apoptosis-specific changes on the cell membrane, ApoSense molecules are designed to enter and accumulate within the dying cell from the early stages of the death process, while being excluded from viable or necrotic cells.
The agent is labeled with the radioisotope F-18 for real-time molecular imaging of programmed cell death with PET.
Related Reading
New growth for diagnostic radiopharmaceuticals, November 9, 2006
Copyright © 2008 AuntMinnie.com