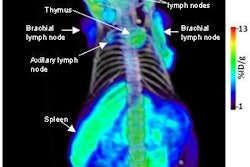
The U.S. Food and Drug Administration (FDA) has designated for priority review GE Healthcare's new drug application for AdreView, a molecular imaging agent for the detection of neuroendocrine tumors in pediatric and adult patients.
The FDA also encouraged the Chalfont St. Giles, U.K.-based company to establish an expanded access program for the agent. An expanded access program is designed to give physicians limited access to a novel agent prior to FDA approval.
GE Healthcare began developing AdreView in 2004.
Related Reading
GE revises Optison's prescribing information, June 11, 2008
GE and UPMC form Omnyx to develop PACS for pathology, June 5, 2008
GE to distribute Kryptiq's ePrescribing package, May 29, 2008
GE launches IT training initiative, May 23, 2008
GE collaborates with CenTrak on RFID technology, May 22, 2008
Copyright © 2008 AuntMinnie.com