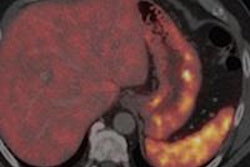
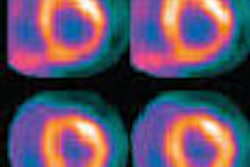
Receptors for the naturally occurring neuropeptide somatostatin have been demonstrated to have a high concentration on neuroendocrine tumors. Indium-111-labeled octreotide (commercially available as OctreoScan by Mallinckrodt of Hazelwood, MO) has long been the preferred agent for imaging somatostatin receptors on neuroendocrine tumors in vivo.
However, a study recently presented by a team of German researchers indicates that there may be a new radiotracer of choice for neuroendocrine carcinoma imaging, Ga-68 DOTA-NOC.
The radiotracer, gallium-68 chloride-labeled macrocyclic chelator DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) with 1-Nal3-octreotide (NOC), is a modification of the octapeptide octreotide. A team from the department of nuclear medicine and PET center at the Zentralklinik in Bad Berka, Germany, has conducted extensive studies with the agent, and found that it enables the molecular imaging of neuroendocrine tumors and their metastases with very high diagnostic accuracy.
"The octapeptide DOTA-NOC has a high binding affinity to the human somatostatin receptor and the potential to target a broader range of somatostatin receptors and concomitantly detect a larger spectrum of tumors," said Dr. Richard Baum, who presented his research team's findings at the 2007 European Congress of Radiology (ECR) in Vienna.
As of October last year, the scientists at the facility had conducted 1,257 Ga-68 DOTA-NOC PET/CT studies on patients with histologically proven neuroendocrine tumors, primarily gastroenteropancreatic, and progressive metastases. These patients were studied before and after peptide receptor radiotherapy, or for follow-up after primary tumor resection, according to Baum.
The patients were injected with an average dose of 100 MBq of Ga-68 DOTA-NOC, and acquisition of images was conducted an average of one hour postinjection. The PET/CT scans were conducted on a Biograph Duo system (Siemens Medical Solutions of Erlangen, Germany).
The group used a protocol similar to what it employs for FDG-PET studies. Scanning started an average of 60 minutes after injection, with two minutes per bed position. For CT scanning they used a low-dose protocol with intravenous and oral contrast.
The group then compared DOTA-NOC uptake in normal tissue to that of cancerous tumors, using standardized uptake values (SUVs). They evaluated SUVs in 12 organs, including the brain, pituitary, thyroid, normal lung, left ventricle, left and right adrenal, intestine, liver, spleen, kidneys, and gluteal muscle.
The group found that SUVs in normal tissue were low, with the highest SUVs reported to be in the spleen (22.04 ± 9.04) and left kidney (12.7 ± 3.65). The lowest SUVs were found in the brain (0.24 ± 0.8) and normal lung (0.75 ± 0.3).
At the same time, DOTA-NOC produced SUVs as high as 152 in tumors, meaning that scans using DOTA-NOC were unlikely to be confounded by background uptake from normal tissue. The group found no uptake in inflammatory processes, although some uptake was produced due to acute infection.
The group also found that the technique produced good sensitivity and specificity -- for example, in the liver, which has a background SUV of 8, DOTA-NOC had sensitivity of 90% and specificity of 89%, Baum said. In one case, the technique found a 6-mm neuroendocrine tumor that had an SUV of 22.
One advantage of DOTA-NOC is that the technique can produce quantitative measurements of tumor volume, and track it over time, such as following successive rounds of therapy, Baum said.
"We think we have an ideal radionuclide ... with high selectivity and low nonspecific binding," Baum said. "I think the advantage is that we have reproducible data which can be used for selecting patients for therapy, we have a fast protocol which is finished after 60-90 minutes, which is patient-friendly, with low radiation burden, and it has much more flexibility than using octreotide with indium-111. We think it's a new gold standard."
By Jonathan S. Batchelor
AuntMinnie.com contributing writer
April 27, 2007
With additional reporting by AuntMinnie.com staff writer Brian Casey.
Related Reading
SPECT/CT enhances octreotide imaging, January 12, 2007
Two studies define role of molecular imaging, therapy in endocrine carcinoma, September 14, 2006
New promise for therapeutic radiopharmaceuticals, June 2, 2006
Copyright © 2007 AuntMinnie.com