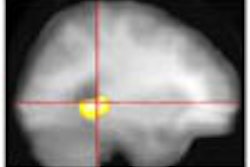
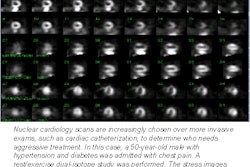
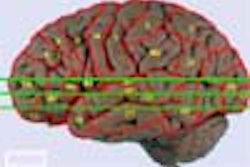
The American College of Nuclear Physicians (ACNP) and the Society of Nuclear Medicine (SNM) on Tuesday said they welcome a statement by the U.S. Office of Management and Budget "that the benefits of regulating medical uses of byproduct materials may not justify the costs of the Part 35 requirements."
The comments were made in a letter to ACNP regarding the Nuclear Regulatory Commission’s proposed revision of 10 C.F. R. Part 35, which governs the medical use of nuclear byproduct materials. The SNM and ACNP oppose the changes proposed by the U.S. Nuclear Regulatory Commission, which would prohibitively raise the cost of nuclear medicine procedures, according to the societies.
The OMB has ordered the Nuclear Regulatory Commission, which proposed the changes, to evaluate its program in an effort to reduce the burden imposed by its requirements.
By AuntMinnie.com staff writers
September 19, 2001
Related Reading
ACNP and SNM fail to modify nuclear medicine regulations, April 18, 2001
SNM, ACNP petition to streamline regulation of byproducts, January 8, 2001
Copyright © 2001 AuntMinnie.com