Perfusion CT can predict the response to radiation and chemotherapy treatment in patients with pancreatic cancer, say researchers from South Korea. The group is hopeful that the results can be used to develop therapies tailored to individual patients, potentially improving survival times while minimizing the toxic side effects of therapy.
There is evidence that radiation combined with chemotherapy can increase median survival time in patients with locally advanced tumors. In addition, neoadjuvant concurrent chemotherapy and radiation therapy (CCRT) may be effective in treating borderline resectable cases of pancreatic cancer, explained Dr. Mi-Suk Park and colleagues from Yonsei University College of Medicine in Seoul in Radiology (January 2009, Vol. 250:1, pp. 110-117).
However, inasmuch as toxicity and disease progression are major obstacles during neoadjuvant CCRT, it would be useful to predict which tumors will respond to treatment and which will not, Park and colleagues wrote. The optimal treatment would be intensive enough to maximize the chance of tumor regression while avoiding unnecessary toxicity.
"Perfusion computed tomography is a clinical technique that can be used to provide regional maps and obtain quantitative measurements of various hemodynamic parameters on the basis of the linear relationship between CT enhancement and iodinated contrast material concentration," and the development of MDCT and perfusion software has facilitated this task, wrote the authors.
The study aimed to "prospectively determine whether perfusion CT parameters, such as the volume transfer constant (Ktrans) between blood plasma and extracellular extravascular space (EES) and the blood volume calculated from dynamic CT data, can be used to predict the response of pancreatic cancer to CCRT," they wrote.
The researchers recruited 63 patients who were scheduled to undergo pretreatment staging of suspected pancreatic cancer. All had adequate renal function (serum creatinine level ≤ 2.0 mg/dL), but 33 patients were excluded for reasons including chemotherapy without radiation (n = 10), surgery without neoadjuvant CCRT (n = 2), cancer other than pancreatic adenocarcinoma at tissue biopsy (n = 11), nondiagnostic CT, a lack of follow-up imaging (n = 5), or tumor less than 1 cm in diameter (n = 5).
The remaining 30 patients with biopsy-proven adenocarcinoma underwent perfusion CT with 64-detector-row CT before CCRT. The treatment included daily external-beam radiation therapy at a planned total dose of 4,500 cGy for 23 patients with tumors in the head of the pancreas and 5,040 cGy for those with cancer in the body and tail of the pancreas (n = 7), administered at 180 cGy along with gemcitabine-based chemotherapy (Gemzar, Eli Lilly, Indianapolis).
Perfusion CT was performed on a Sensation 64 scanner (Siemens Healthcare, Erlangen, Germany). The tumor was localized at noncontrast CT, followed by a dynamic exam of the target region performed at end expiration. Iodinated contrast (50 mL iopamidol, Bracco, Milan, Italy) followed by 50 mL saline was injected at 4 mL/sec via a power injector (EnVision CT, Medrad, Warrendale, PA).
Four contiguous 7.2-mm thick sections were acquired at one-second intervals through the midpoint of the tumor by using a cine-mode acquisition (100 kVp, 100 mAs, 4-mSv effective dose). CT scanning was initiated five seconds after the start of injection.
Two minutes later, following injection of less than 150 mL of nonionic iodinated contrast, pancreatic and portal-phase dynamic CT were performed at 40 and 60 seconds, respectively, after contrast media injection. Parameters included 120 kV, 200 to 250 mAs effective dose, 64 x 0.6-mm collimation, 3-mm image thickness, and 3-mm increment reconstruction.
For follow-up CT at three and six months, two-phase dynamic CT scanning was performed without perfusion CT to evaluate treatment response. The researchers compared two perfusion parameters (Ktrans and blood volume) measured before treatment, comparing responders to nonresponders according to World Health Organization criteria.
Of the 30 patients, 20 (mean age, 66.6 years; age range, 55-79) examined at three months responded to therapy, with bidimensional tumor size reductions of at least 50%. Ten patients (mean age, 65.8 years; age range, 53-77) who did not meet this criterion were deemed nonresponders.
Responders' pretreatment Ktrans values were significantly higher than those of nonresponders (50.8 mL/100 mL/min ± 30.5 standard deviation versus 19.0 mL/100 mL/min ± 10.8, p = 0.001). "The best cutoff value for differentiating between responders and nonresponders was 31.8 mL/100 mL/min, which yielded 75.0% sensitivity and 90.0% specificity," the group reported.
Ten of 18 patients examined at six-month follow-up had responded to therapy. Similar to the three-month follow-up, their pretreatment Ktrans values were significantly higher than those of nonresponders (58.6 mL/100 mL/min ± 43.2 versus 19.8 mL/100 mL/min ± 10.9, p = 0.02). Responders also had insignificantly higher blood volume values.
The baseline higher Ktrans values seen in responders "could indicate better delivery of the chemotherapeutic drug to the tumor," the authors wrote. "Gemcitabine, which was the main chemotherapeutic drug used in our study, is a cytotoxic agent that targets DNA and RNA and acts as a radiosensitizing agent. Thus, increased delivery of gemcitabine may induce an increased radiosensitizing effect and an increased cytotoxic effect, resulting in good response to CCRT."
 |
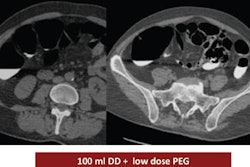
| Images obtained in a 63-year-old man with locally advanced pancreatic head cancer who was a member of the responder group. Above, transverse contrast-enhanced baseline CT image shows a locally advanced pancreatic head cancer mass. The long-axis diameter of the mass was 48.0 mm, and the perpendicular diameter was 22.2 mm. The bidimensional product was 1,016 mm2. At that time, the CA 19-9 level was 3,130 U/mL. |
 |
| CT image obtained three months later at first follow-up shows partial response of the pancreatic head cancer mass, which decreased in size to 388 mm2. The CA 19-9 level was 409 U/mL. |
 |
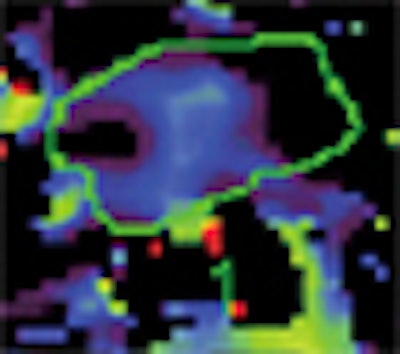
| Baseline perfusion map reveals a Ktrans value of 53.8 mL/100 mL/min, which is higher than the best cutoff value (31.8 mL/100 mL/min). The scale does not include the peripheral portion of the tumor (red). This case shows three zones, two of which have high perfusion (left small and right large areas) and one that has low perfusion (central area). Presumably, this reflects the fact that pancreatic cancer may have heterogeneous perfusion even though it appears to be a homogeneously enhancing mass on conventional contrast-enhanced CT images. |
 |
| Baseline blood-volume perfusion map shows a volume of 4.4 mL/100 mL, which is higher than the best cutoff value (2.8 mL/100 mL). All images republished with permission of the Radiological Society of North America from: Park MS, Klotz E, Kim MJ, et al. Perfusion CT: Noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology. 2009;250(1):110-117. |
Only a few studies have sought to evaluate the value of perfusion CT as a therapeutic monitoring method in patients with tumors. Among them, Sahani and colleagues found that rectal cancer patients with initial high blood flows and shorter mean transit times showed a poor response to CCRT, the authors noted.
Another study, by Zima and colleagues, examined patients with upper aerodigestive squamous carcinomas with perfusion CT. Initial high blood flow, high blood volume, and high capillary permeability were correlated with a good response to chemotherapy. The results suggested that higher perfusion values may correlate with better oxygen and drug delivery.
Limitations of the study included radiation exposure in the range of 4 mSv, and a smaller sample size than anticipated because several studies had to be discarded for poor image quality. The authors also noted the complexity of scanning patients with abdominal tumors, imperfect breath-holding, and artifacts from intra-abdominal air, which limit the quality of the voxel time-attenuation curves that are entered into the mathematical models of the evaluation software.
"The small enhancement of pancreatic carcinoma further reduces the signal-to-noise ratio," they wrote. The Patlak method used in the study is fairly robust and needs few data points, which enabled scanning time to be reduced to 30 seconds.
"Pancreatic tumors with high pretreatment Ktrans values indicating higher intratumoral flow tended to respond better to the gemcitabine-based CCRT," the researchers concluded. Pending the completion of larger studies, "perfusion CT may be used to predict the tumor response of CCRT in patients with pancreatic cancer and to aid in the development of tailored therapy."
By Eric Barnes
AuntMinnie.com staff writer
January 2, 2009
Related Reading
CT perfusion scan spots acute stroke in the emergency setting, November 5, 2008
New neuroimaging potential seen in 320-row CT, October 20, 2008
High blood flow to breast cancer tumors linked to high recurrence risk, October 16, 2008
ROI changes colorectal tumor perfusion measurements, August 26, 2008
Study tests low dose ranges for perfusion CT, December 10, 2007
Copyright © 2009 AuntMinnie.com