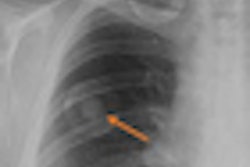
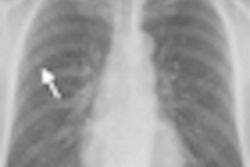
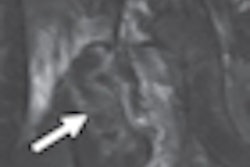
Computer-aided detection (CAD) developer Riverain Medical of Dayton, OH, has received clearance from the U.S. Food and Drug Administration (FDA) for the newest version of its OnGuard chest x-ray CAD technology.
The new version demonstrates a 73% reduction in false-positive marks and a 50% higher relative sensitivity compared to the original product, according to Riverain.
Related Reading
Riverain wins Veterans Affairs contract, June 17, 2010
Riverain nets FDA OK for SoftView, March 25, 2010
Riverain scores Canadian sales deal, November 19, 2009
Riverain scores regulatory approvals, November 19, 2008
Road to RSNA, CAD, Riverain Medical, October 30, 2008
Copyright © 2010 AuntMinnie.com