Reimbursement is critical for the success of any new imaging technology. Adoption of chest x-ray computer-aided detection (CAD) may have received just that boost following a recent release of an American Medical Association (AMA) Current Procedural Terminology (CPT) code and a subsequent published non-Medicare resource-based relative value scale (RBRVS) code.
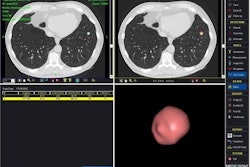
Less than a year after Riverain Medical debuted its RapidScreen chest x-ray CAD system at the 2004 RSNA meeting in Chicago, the AMA issued a Category III CPT code covering chest x-ray CAD. Code 0152T, which becomes effective January 1, 2006, is broadly worded:
0152T Computer-aided detection (computer algorithm analysis of digital image data for lesion detection) with further physician review for interpretation, with or without digitization of film radiographic images; chest radiograph(s) (List separately in addition to code for primary procedure)(Use 0152T in conjunction with 71010, 71020, 72021, 71022, and 71030).
In October 2005, a non-Medicare fee schedule was published for this Category III CPT code. This is the first time that a radiological Category III CPT code has received a published non-Medicare RBRVS code, according to Michael Longacre, a consultant with HealthCare Market Strategies of Yamhill, OR.
Longacre attributes this radiology reimbursement milestone to lung cancer's role as the leading cause of cancer deaths in the U.S. An estimated 165,310 people are expected to die of lung and bronchus cancer in 2005, based on statistical projections from the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC).
The cost of treating lung cancer is staggering, more than $5 billion in 1996. The survival prognosis after diagnosis and treatment is grim. Individuals with stage III and IV lung cancer have less than a 15% chance of survival after five years. Of the estimated 172,570 individuals who will be diagnosed with lung cancer in 2005, only 16%, or 27,600 people, will be detected at stage I or II. After treatment, their odds of survival for five years rise to 49%.
Longacre believes that the potential benefit of CAD analysis of x-rays has been acknowledged by the medical payor industry, and that the procedural bar to attain financial reimbursement for new, FDA-approved technology utilization has been lowered.
With the combination of the Category III CPT code and the publishing of a non-Medicare RBRVS, providers can report the usage of CAD with chest radiographs, their usage will be documented in a database, and negotiations with payors can be initiated based on published reimbursement fees.
Category III CPT codes are established by the AMA to facilitate data collection on and assessment of new services and procedures. They are intended to be used to substantiate widespread usage, and are terminated after five years.
The language of the code has been written to accommodate new chest CAD algorithms that will be developed to detect such conditions as pneumothoraces, cardiac enlargement, and interstitial lung disease, said CAD researcher Dr. Matthew Freedman, director of the Division of Advanced Cancer Imaging at Georgetown University's Imaging Science and Information Systems Research Center.
"In my opinion, the AMA indirectly stated that this is a technology of the present and the future," said Freedman, who's been working with CAD technology since 1991. "The code will extend far beyond Riverain's current product as new applications for chest CAD are developed."
The new developments will move the utilization of CAD with chest imaging forward, as there's now the potential of reimbursement for providers, said fellow CAD researcher Dr. Heber MacMahon. MacMahon is the associate director of the Kurt Rossman Laboratories for Radiological Image Research at the University of Chicago. MacMahon; Kunio Doi, Ph.D., professor and director of the Kurt Rossman Laboratories; and the affiliated research team have participated in the development of CAD schemes for detection and differential diagnosis on radiological images for the past 20 years.
"Particularly at this early stage of development, radiologists need some incentive to make the effort to implement CAD for chest radiographs and to explore what its potential really is," MacMahon said.
Freedman and MacMahon, both of whom are affiliated with Riverain Medical as consultants, believe that radiologists of all levels of experience can benefit from chest CAD utilization.
"A tremendous amount of research has been conducted over the past 30 years on the accuracy of radiologists and the occasional failure to detect significant abnormalities that are visible in retrospect," MacMahon said. "The potential for this technology is to bring abnormalities to the radiologist's attention so that he or she will focus on them or at least consider whether they will need further investigation."
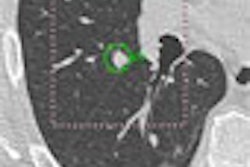
In a clinical trial of RapidScreen performed at Georgetown's Imaging Science and Information Systems Research Center in 2003, the impact of CAD in detecting early-stage cancer varied significantly among individual radiologists (Proceedings of the 19th International Congress and Exhibition: Computer Assisted Radiology and Surgery, Berlin, Germany, June 22-25, 2005, pp. 1087-1092).
However, 10 out of the 15 highly competent community hospital-based radiologists participating in the clinical trial benefited from using the technology, Freedman said. This group included those with the least amount of thoracic imaging experience and those with the most.
Freedman believes that at this stage of technology, variability is to be expected. He said that the algorithm's performance is based on its interpretation and translation of methodology used by expert thoracic radiologists and also from a very detailed mathematical analysis most effectively performed by a computer.
"This combination can find potential abnormalities that a radiologist may not identify at the time of image interpretation, but will recognize when pointed out by the computer," Freedman said.
Both Freedman and McMahon believe that chest CAD technology is a very useful training aid for residents to become more proficient at identifying abnormalities that may identify lung cancer.
Reimbursement also helps. Riverain reports that since July 2005, when the AMA announced the CPT code, the number of inquiries to the company and requested evaluations soared.
By Cynthia Keen
AuntMinnie.com contributing writer
November 22, 2005
Related Reading
Riverain brings Deus lung CAD to market, December 20, 2004
X-ray CAD finds bone defects in rheumatoid arthritis, May 18, 2004
Copyright © 2005 AuntMinnie.com