CT may not be the perfect test for acute pulmonary embolism (PE), but it is arguably the best way to rule out an emergent and chronically underdiagnosed condition that strikes 600,000 Americans a year, killing perhaps a quarter of them within a year.
Even single-slice scanners have demonstrated sensitivities on the order of 85% for the detection of pulmonary artery filling defects that define PE. And over the past decade, multidetector-row machines have added the detection of subsegmental emboli, though blind spots remain in CT's ever-sharpening eye.
In a talk at the 2004 International Congress of Radiology in Montreal, Dr. John Mayo, associate professor of radiology at the University of British Columbia and head of general radiology at Vancouver General Hospital in British Columbia, Canada, discussed CT's increasingly dominant role in PE detection, delved into the nitty-gritty of image interpretation, and shared new developments in its use, from CT venograms to the rise of perfusion maps and CAD.
Interpretation was actually easier before the multidetector-row scanners came along, he said.
"On single-slice scanners it's great because after you've gone through the central pulmonary arteries and all the segmental arteries, it's like the day is done -- it's Miller time," Mayo said. But the search for the telltale vascular filling defects of PE takes longer on multislice scanners. "You can analyze ever further out there on subsegmental pulmonary arteries as you go to more simultaneous tracks, and as you go to a faster rotation time," he said.
Still, MDCT is no panacea. It cannot adequately analyze distal vessels in the capillary bed because of spatial resolution issues, for example. "There are going to be vascular diseases at the periphery of the lung that are going to full well remain the domain of the pathologist for a long time to come," Mayo said.
Ghaye and colleagues demonstrated that thin-section (1.25-mm) reconstruction allowed the analysis of significantly more subsegmental arteries, Mayo noted (Radiology, June 2001, Vol. 219:3, pp. 629-636).
And a study presented at the 2003 RSNA meeting in Chicago found that thin-section reconstructions also improved the confidence of the diagnosis (Abstract No. 320, Sheehan RE, Melsom SM, Muller NL, Mayo JR).
Scanning for PE
A principal technical goal of CT pulmonary angiography (CTPA) is to generate the thinnest slices possible on the available equipment, Mayo said. On a single-slice scanner, thin means 3-mm collimation and the standard reconstruction algorithm, resulting in a voxel size of 1 x 1 mm in the x and y axes. With a 64-slice scanner, images can be acquired at 0.625 mm x 0.625 mm x 0.625 mm. Collimation of 1 mm or 1.25 mm produces a standard dataset of 300-400 images to cover the chest.
Excluding PE with multislice CT also means scanning the entire lung. With single-slice CT, the search is limited to the central and segmental pulmonary arteries -- one can get away with scanning from the aortic arch down to about 1 cm below the lowest hemidiaphragm, Mayo said. But MDCT adds subsegmental arteries to the quest, requiring a full lung scan from apex to base.
Crucial contrast
Contrast enhancement is as crucial as thin sections. The goal is to obtain relatively dense contrast enhancement of the blood -- a minimum of 200 HU in the central pulmonary artery.
"We achieve that level of enhancement by injecting contrast at 3-4 cc per second for a total volume between 100-150 mL for single-slice scanners, and one can get down to as little as 80 mL for a multitrack scanner," while still administering at a rapid rate of 3-4 cc per second, Mayo said.
Sufficient contrast must be delivered to the region of interest to obtain significant difference in attenuation between clotted blood and flowing blood, he said. Without the density difference, the radiologist cannot hope to detect an embolus. Contrast delivery is a particular concern among young patients due to high cardiac output, he said.
"The (contrast) density in the blood is strictly related to the cardiac output divided by the rate of contrast administration," he said. "So in very young and anxious patients, it may be useful to up the rate of contrast delivery to 4 or even 5 cc per second if ... you've had a previous nondiagnostic scan."
Follow the vessels
Large MDCT datasets -- a chest scan at 1-mm collimation will comprise 300-400 images -- make scrolling crucial.
"When one interprets those images on film, it's very difficult to go vessel to vessel to vessel," Mayo said. "However, if you put them on a workstation and scroll back and forth, you can focus on the pulmonary artery and identify it as it branches out to the peripheral lung, and that's the real reason you should interpret these with a scrolling technique."
Mayo's group begins the interpretation with narrow window settings of 300-400, level 35, increasing the width to 600 (level 100) to see the small peripheral vessels. The wider setting also allows examination of the contrast column, to look for small clots that might otherwise be masked by the dense contrast. Finally, it's important to review the lung parenchyma at the lung settings to look for ancillary findings, he said.
"Acute PE diagnostic findings are really quite straightforward, and are the CT equivalent of pulmonary angiographic findings," he said. "They include intraluminal filling defects and vascular cutoff -- and nothing else counts. It's important to actually see the embolus within the vessel, not just sort of say, 'Well, I think I can see it,' or 'I don't see this vessel anymore.'"
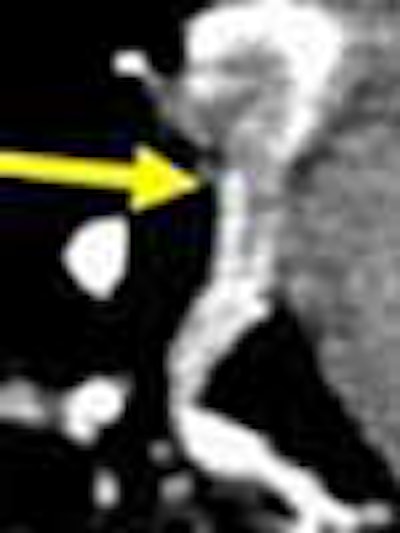
 |
| Intraluminal filling defects reveal pulmonary embolism in target sign (above), and tram track sign (below). Images courtesy of Dr. John Mayo. |
 |
Once a clot is spotted, radiologists must test the theory by asking themselves how the alleged embolus is able to remain immobile, while the pulmonary vasculature goes into high flow "like a toilet flushing," once per second, he said. Is the "embolus" immobile because it's hooked into a vessel? If not, it might be an artifact. True clots should have a visible means of escaping the blood flow. Similarly, he said, a "clot" that appears on just one or two sections on both the right and left sides might be a flow artifact.
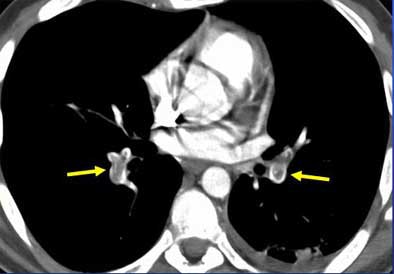
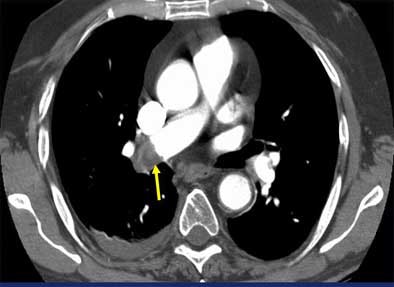 |
| Vascular cutoff sign. Image courtesy of Dr. John Mayo. |
Moreover, a suspected saddle embolus doesn't justify a pulmonary angiogram, though surgeons and internists often seem to think it does, Mayo said. For one thing, a pulmonary angiogram is difficult to do in the presence of a large saddle embolus because it results in a dilated right ventricle. For another, saddle emboli create a significant flow blockage that is easily detectable on CT, he said.
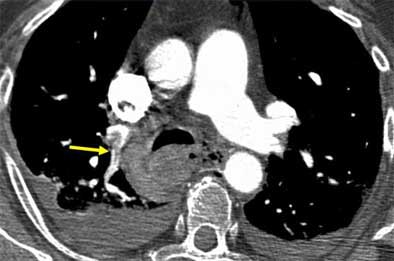
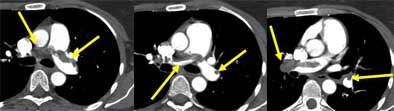 |
| Saddle embolus. Images courtesy of Dr. John Mayo. |
Suspicious signs
Findings that are suggestive but not diagnostic of pulmonary embolism include dilated central pulmonary arteries, wedge-shaped pleural-based consolidations, and dilatation or evidence of pressure overload of the right ventricle, he said.
"(With a) PE sitting in the vessels, quite often we will notice that the vessels on the side that has extensive clot ... are significantly larger than those ... seen on the (other) side," Mayo said. "Anytime you have asymmetric enlargement of the vessels, have a good look on the inside of the vessels to see ... that there's not a clot sitting inside the vessel itself."
Thus are emboli differentiated from nodes outside the vessels. Reformatted images can occasionally be helpful in determining the precise location of such filling defects. Wedge-shaped peripheral densities are also common, especially in the lower lobes, he said. They can appear as either ground-glass densities or frank consolidation.
"Some people have called these (wedge-shaped densities) all pulmonary infarcts, but we see many more of these than we believe are infarcts, and in follow-up some of them completely resolve ... with no sequelae," Mayo said. "So they likely represent degrees of pulmonary hemorrhage, and some of them may go on to represent pulmonary infarcts, but not all." Pulmonary infarcts are associated with thrombosis on the venous side of circulation, he said.
Dilatation and/or straightening of right ventricle is also suspicious, he said. The intraventricular septum isn't held in its curved configuration by any bony or ligamentous structures, so its shape can be altered by hemodynamic pressure differences between the right and left sides.
"In those situations we always get dilatation of the right ventricle, the tricuspid valve annulus dilates, and we get acute tricuspid regurgitation and enlargement of the right atrium as well," he said. "Sometimes we can also see contrast refluxing down into the hepatic veins."
Interpretive challenges
Interpretive pitfalls include motion artifacts, suboptimal contrast injection, limited signal noise, and hilar lymph nodes, Mayo said.
The "star" artifact, seen as radiating areas of high and low density, occurs when the image-processing reconstruction algorithm assumes that the heart is motionless over the course of the scan, he said. Interpolation artifacts, which can also cause interpretation problems, are diminished in scanners with better spatial resolution. Suboptimal contrast injection and noise are additional problems, as are bilateral pearl effusions because they eat up lots of photons, he said.
When contrast enhancement is suboptimal and there are marked effusions, it often makes sense to drain the effusions first, as they frequently cause patients to present with shortness of breath, Mayo said. But at that point the question of PE may remain unresolved, so adjusting the contrast bolus and rescanning may be needed for better contrast opacification.
Still, a few cases will not improve after draining the effusions. The problem may be due to patients taking a deep breath before the scan. This can produce a dilution of contrast density because large amounts of nonopacified blood flow in from the vena cava, diluting contrast density in the pulmonary outflow track. Sometimes rescanning with the patient breathing shallowly can solve this problem, Mayo advised.
"If you need to (rescan), the recognition that it's artifact and not bilateral symmetric PE is a) that the emboli can't sit there because they're not hooked into any vessel, b) (they) are not well-circumscribed, and c) (they) occur in bilaterally symmetric fashion, which is very unusual for PE," he said.
Inexperienced readers often make the mistake of calling PE on pseudovascular cutoffs caused by secondary nodes, commonly seen in the transverse section. Scroll inferiorly a bit and the "emboli" will often disappear, he said.
In any case, the literature shows a 2% to 4% rate of suboptimal CT exams, in line with the 3% rate reported for suboptimal pulmonary angiograms. They tend to occur in the same patients in both modalities, he said, in those "who are anxious, won't hold their breath, and are shuffling around on the angio table and the CT scanner table."
A few good studies
As for choosing CT as a front-line exam, several studies have compared it with scintigraphy, Mayo said. In one, Cross and colleagues randomized 78 patients to ventilation/perfusion scintigraphy (V/Q scan) or CTPA. The main difference was that CT diagnosed 35/39 patients (90%), compared to 21/39 patients (54%, p < 0.001) randomized to V/Q scan. CT was also deemed superior for its ability to diagnose many of the 18 remaining cases that were negative for PE (Clinical Radiology, March 1998, Vol. 53:3, pp. 177-82).
Radiation concerns make direct CT-to-angio studies hard to come by, but in 2000, Baile and colleagues injected acrylic "emboli" into pigs, and subsequent examination of the lungs found CT and pulmonary angiography to be equivalent for the detection of PE. Sensitivity and 95% confidence intervals for 3-mm and 1-mm collimation CT and angiography, respectively, were 82% (73% to 88%), 87% (79% to 93%), 87% (79% to 93%) (p = 0.42), the authors wrote (American Journal of Critical and Respiratory Care Medicine, March 2000, Vol. 61:3, pp. 1010-1015).
Still, CT adds advantages, including visualization of the contrast column, Mayo said.
Kim et al found that spiral CT suggested or confirmed an alternate diagnosis in patients who were proved not to have PE, and in doing so produced useful information in 80/110 patients, Mayo said (Radiology, June 1998, Vol. 207:3, pp. 753-758).
"I believe the real reason spiral CT has become so widely employed in query of acute PE is because even in the best series, only 30% with a question of acute PE actually turn out to have PE," he said. "But all of them have significant chest symptoms; that's the reason they came to clinical attention to begin with. CT is a very useful general-purpose diagnostic tool, and in doing a CT pulmonary angiogram you get a very high-quality CT scan of the chest as well."
Still, studies have shown a wide range (6% to 30%) of disagreement for findings of subsegmental emboli, probably related to patient selection, and epidemiologists have questioned the generalizability of CT findings (Journal of the American Medical Association, May 23-30, 1990, Vol. 263:20, pp. 2753-2759; Radiology, April 1996, Vol. 199:1, pp. 31-35).
However, the results from the second part of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED II) study coming in 2005 are expected to provide definitive answers, Mayo said.
Adding heft to CT's clinical value are two recent studies showing low rates of recurrent PE (1.8% and 1.5%) in patients who had a negative CT and ultrasound studies (The Lancet, August 2003, Vol. 362:9383, pp. 523-526; December 14, 2002, Vol. 360:9349, pp. 1914-1920).
"And that has led one of the great Canadian skeptics of this whole thing, Dr. (Clive) Kearon ... to state in an editorial that the evidence is catching up with the enthusiasm, which I took as a personal compliment," Mayo said (Canadian Medical Association Journal, May 2003, Vol. 168:11, pp. 1430-1431).
New issues
The literature suggests that spiral CT is 85% to 90% sensitive and 90% to 95% specific for PE, and that filling defects seen in CT data are truly pulmonary emboli, Mayo said. Single-slice CT does central and segmental pulmonary arteries quite well, while multislice CT adds subsegmental emboli to the diagnosis, although the clinical significance of the smallest emboli is still being evaluated, he said.
"The addition of CT venography to CTPA is being hotly debated right now, and it will take further investigation to determine its exact role," he said. "There are issues with regard to expense, issues with regard to radiation exposure, and I think this requires more study ... so we are not routinely doing CT pulmonary venograms following a CTPA exam. We certainly wouldn't consider it for someone under the age of 40; we do it in certain instances above the age of 40 when patients have a (large) body habitus that renders them poor (candidates) for doing Doppler US measurements."
Pulmonary perfusion maps are tantalizing as well, he said, but their principal problem is a doubling of the radiation dose to produce a noncontrast scan needed in addition to the regular contrast scan. All things considered, the incremental gain in sensitivity from perfusion maps seems quite small inasmuch as CTPA is already highly sensitive and specific, he said.
As for CAD, it is an important field of pursuit, and can be useful for community-level radiologists who deal with fewer cases of PE than the specialists, and are perhaps less familiar with the branching patterns of pulmonary vasculature, Mayo said. But current CAD systems are calling too many (false-positive) emboli in the subsegmental vessels.
"(CAD systems) are great in central and segmental vessels that are sort of a slam dunk for anyone, and they're helpful so you don't overlook an obvious embolus -- good from a second-reader point of view," he said. "But where you have limited image quality and motion, for the most part our experience is that they end up asking more questions than they answer."
By Eric Barnes
AuntMinnie.com staff writer
September 10, 2004
Related Reading
MDCT drives important changes in U.K. healthcare, August 10, 2004
Combined test strategy safely detects pulmonary embolism in outpatients, March 11. 2004
MDCT pulmonary angiogram rules out PE for months, March 22, 2004
Volumetric capnography points to pulmonary embolism, March 17, 2004
CT assessment of pulmonary embolus predicts prognosis, March 16, 2004
Troponin I test adds to prognostic value of echo in acute pulmonary embolism, October 27, 2003
D-dimer testing can eliminate need for ultrasound in patients with suspected DVT, September 25, 2003
Copyright © 2004 AuntMinnie.com