The results come from a phase I clinical trial of radium-223 for treating patients with high-risk relapsed bone-forming osteosarcoma.
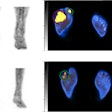
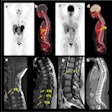
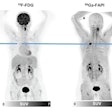
The 18 patients in the study -- all of whom had multiple lesions -- received one to six cycles of radium-223, with doses ranging from 6.84 MBq to 57.81 MBq. The researchers, led by Dr. Kalevi Kairemo, PhD, from MD Anderson Cancer Center, used bone scintigraphy, FDG-PET, or F-18 NaF PET to further characterize the disease.
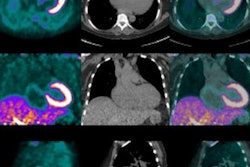
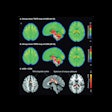
The studies could be compared in 10 patients at two time points, with the number of lesions and their locations and volumes analyzed using FDG-PET and NaF-PET.
Kairemo and colleagues used a novel imaging and evaluation technique called NAFCIST; it is similar to the PET Response Criteria in Solid Tumors (PERCIST), but based on NaF-PET results.
Given the preliminary findings, the group believes NAFCIST could become a new tool in response evaluation for high-risk osteosarcoma cases after radium-223 treatment.
"NaF demonstrates soft-tissue metastases in osteosarcoma (lung, liver, brain, lymph node), and five lesions in multiple organs can be found," they wrote. "Our results indicate that NaF-PET is an essential part of osteosarcoma staging and NaF-PET and FDG-PET are complementary in osteosarcoma."