Post-Transplant Lymphoproliferative Disorder (PTLD):
(See also: Complications of Lung Transplantation)Clinical:
Immunosuppression in transplant patients increases their risk of post-transplant neoplasia- specifically these patients are at increased risk for developing lymphoproliferative disorders ranging from benign polyclonal hyperplasia to malignant non-Hodgkins lymphoma. Lung, heart, and pancreas transplant patients require higher doses of immunosuppression (compared to kidney and liver transplants) and have a higher risk of PTLD [9]. Most post-transplant lymphoproliferative lesions are B-cell phenotype, but about 14% of lesions are T-cell [5,7]. The disorders tend to occur within 1 year after transplantation (average 4 months) and usually regress after a decrease or cessation of immunosuppressive therapy (cyclosporin) and the administration of antiviral agents (acyclovir). Chemotherapy is used for lesions that do not respond to immunosuppression reduction and also for monoclonal lesions [7]. Rituximab is also being used for treatment of PTLD [7]. Early onset following transplant (within the first year and accounting for 80% of cases) and localized disease predict a better prognosis. Late onset disease (after 1 year) has a 70% mortality rate. Definitive diagnosis requires pathologic confirmation.There is a correlation between the cumulative dose of immunosuppressive therapy and the incidence of PTLD. Most cases of PTLD have been associated with concomitant Epstein-Barr virus (EBV) infections and this may be the etiologic agent, however, PTLD can also be seen in EBV negative patients (more often these patients have later onset PTLD beyond one year) [7]. Patients that are seronegative for EBV pre-transplant are at higher risk for developing PTLD compared to those that were seropositive [5]. Up to 93% of post-transplant patients with PTLD are found to be EBV positive [5]. However, it is important to remember that 60% of all transplant recipients may have evidence of EBV infection, but only PTLD develops in only a small percentage of these cases [5]. The Ebstein-Barr virus induces polyclonal B-lymphocyte proliferation. Normally, B-lymphocyte proliferation is controlled by suppressor T-cells, however, these cells demonstrate diminished function in immunosuppressed transplant recipients [8]. This allows the B-lymphocytes to proliferate unchecked and an aggressive or malignant B-lymphocyte clone may predominate. [1]
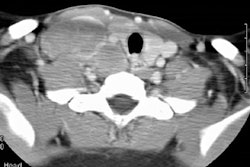
The frequency of PTLD varies with the type of organ transplant [8]. PTLD develops in about 1-2% of bone marrow transplant patients, 1% of renal transplants, 2% of liver transplants, 2-8% of lung transplant patients, in 5 to 20% of heart/heart lung transplant patients, in 7-11% of bowel transplant patients, and in 13-33% of multivisceral transplant recipients [7]. The higher prevalence of early PTLD in bowel, lung, and lung-heart transplant recipients us felt to be related to more aggressive immunosuppression/antirejection therapy in these patients [7]. The anatomic site of PTLD is most often the allograft itself [7]. The abdominal cavity is a frequent site of disease and extranodal involvement is seen in about 80% of cases [7]. The liver is the most frequently involved solid abdominal organ (site of involvement in 30-45% of liver transplants, 40% of pancreas transplants, 23% of heart transplants, and 10% of post lung transplant PTLD's) [7]. If the bowel is involved, the most common site is the distal jejunum and ileum [7].
Thoracic involvement is the site of PTLD in 69-100% of lung transplant patients, 80% of lung-heart transplant patients and 32% of heart transplant patients; however, overall thoracic involvement is less common than abdominal disease [7].
Children are more likely to develop PTLD than adults [8]. In children, PTLD may have a tendency to develop in the anatomic region of the transplant/allograft organ- possibly related to antigenic stimulation from the organ itself [6]. Also- prognosis may be more related to the patients underlying immune dysfunction, rather than the extent of disease (ie: localized versus multiorgan) [6].
X-ray:
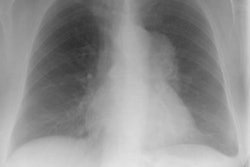
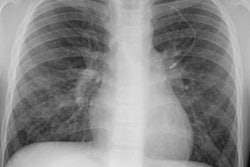
Multiple (more commonly) or solitary pulmonary nodules or mass/es ranging in size from 3 mm to 5 cm are the most common pulmonary manifestation in cases of PTLD. The margins may be smooth or irregular. A solitary pulmonary nodule is associated with a more favorable course [7]. The nodules can have a peripheral ground-glass halo which can mimic aspergillosis infection [8]. Mediastinal and hilar adenopathy can also been seen in 7-50% of cases. Patchy airspace consolidations (7-10% of cases), areas of ground-glass attenuation, and pleural effusions have also been described in association with PTLD. Radiographic findings can mimic infection or BOOP. Rarely, PTLD may appear as a diffuse reticulonodular infiltrate.The lesions of PTLD are typically FDG avid [8].
REFERENCES:
(2) Radiol Clin North Am 1994 Jul;32(4):663-678
(3) AJR 1997; Radiologic manifestations of lymphoma in the thorax. 168: 93-98 (no abstract available)
(4) AJR 1997; Imaging of pulmonary lymphomas.168: 339-345 (no abstract available)
(5) Radiology 1998; Collins J, et al. Epstein-Barr-virus-associated lymphoproliferative disease of the lung: CT and histologic findings. 208: 749-759
(6) AJR 1998; Donnelly LF, et al. Lymphoproliferative disorders: CT findings in immune compromised children. 171: 725-731
(7) Radiographics 2009; Borhani AA, et al. Imaging of
posttransplant lymphoproliferative disorder after solid organ
transplantation. 29: 981-1002
(8) Radiographics 2016; Sirajuddin A, et al. Primary pulmonary
lymphoid lesions: radiologic and pathologic findings. 36: 53-70
(9) Radiographcis 2016; Katabathina VS, et al. Malignancy after
solid organ transplantation: comprehensive imaging review. 36:
1390-1407