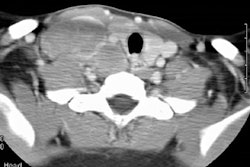
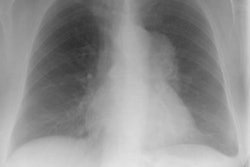
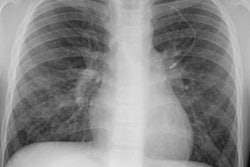
Lymphoma:
Residual abnormality following completion of therapy:
See also discussion of FDG PET imaging in lymphoma
Up to two-thirds of patients with initial mediastinal involvement will have persistent radiographic mediastinal abnormalities following completion of therapy- yet only 18% of these patients ever relapse. Conventional imaging modalities must generally rely upon an increase in size of a residual mass as a marker for recurrence [8]. Thymic hyperplasia following chemotherapy may create confusion by mimicking recurrence, but it is usually not seen until several months after completion of chemotherapy [1].
Following therapy, CT is unable to distinguish residual tumor from fibrous tissue.
Scintigraphy in residual mass evaluation:
Nuclear scintigraphy with Ga-67 has shown tremendous use for this purpose. Since gallium is a viability agent, it is taken up by cancer tissue and not by fibrotic or necrotic tissue at the tumor site. When scanning patients for recurrence it is essential to image the entire body since about 25% of patients will relapse only in new disease sites different from the primary site of involvement [2,3].
Chemotherapy may suppress gallium uptake for some time after treatment in spite of active disease. At least a 3 week delay after completion of chemotherapy is recommended prior to re-imaging. Tumor irradiation may also result in transient or permanent loss of gallium uptake. When gallium uptake is observed in a residual mass indicating viable tumor following treatment, further non-cross resistant treatment should be instituted. The presence of residual gallium uptake after treatment has been shown to be a poor prognostic indicator in patients with Hodgkin's and non-Hodgkin's lymphoma. In Hodgkin's patients overall survival decreased from 100% to 60% at 2 years in patients with positive scans at the end of treatment and decreased from 90 to 35% in non-Hodgkin's patients [3]. Ga-67 can also be used during treatment of Hodgkins or non-Hodgkins lymphoma to monitor response to therapy and direct changes in treatment if scans remain positive [6,7].
Frequently, bilateral hilar nodal activity can be seen in patients that have completed chemotherapy and this activity may persist for over 4 years on follow-up exams. This uptake is only very rarely associated with the presence of residual lymphoma. It may be related to the presence of a concomitant inflammatory disease or possibly a direct effect of the chemotherapy. Unilateral hilar uptake post therapy is somewhat more concerning and should raise the possibility of recurrent disease. If necessary, thallium imaging may be useful in these instances as it will accumulate within tumor involved nodes, but will be negative in the absence of disease.
Diffuse lung uptake of Ga-67 has also been described following treatment, occurring in up to 12% of patients. The uptake is most commonly mild and does not indicate lymphomatous involvement of the lungs. The uptake most likely reflects pulmonary toxicity secondary to chemotherapy [4].
MRI in residual mass evaluation:
Magnetic resonance imaging has also been used to evaluate residual masses following completion of therapy. For the prediction of relapse in a residual mass, MR imaging has a sensitivity of 45-90% and a specificity of 80-90% [8]. A homogeneous or heterogeneous low signal intensity on T2 weighted images is generally felt to be compatible with a fibrotic mass with no residual tumor. Unfortunately, the presence of low signal on T2 images does not exclude the possibility of recurrence [1,5,8]. Areas of increased signal on T2 images are felt to be considered suspicious for recurrence. However, this finding may also be seen with intratumoral necrosis (particularly when identified within 6 months of completion of chemotherapy), immature fibrotic tissue, edema, retained secretions in adjacent radiated lung, and fat interspersed with fibrosis [8]. Gadolinium enhanced MR imaging has also be used to evaluate for the presence of tumor recurrence [8]. Compared with a baseline exam, decreased contrast enhancement should be seen following successful therapy [8]. Any changes in the enhancement pattern should be considered suspicious for recurrence [8]. Unfortunately, there is a large amount of variability in the degree of decreased enhancement following therapy, and no specific quantification of the degree of enhancement for confirmation of recurrence has yet been determined [8]. Drawbacks of MRI in the evaluation of residual masses include the fact that only a small number of patients have been evaluated, biopsies were not obtained in all patients, and small foci of tumor may not be morpholgically detectable.
REFERENCES:
(1) Radiology 1997; 203: 369-376
(2) J Nucl Med 1993; 34(12), 2101-2104
(3) Semin Nucl Med 1995 Jan;25(1):60-71
(4) Radiology 1996; 199: 473-476
(5) Radiology 1993; 188: 445-451
(6) Radiology 2000; Front D, et al. Aggressive non-Hodgkin lymphoma: Early prediction of outcome with Ga67 scintigraphy. 214: 253-257
(7) Radiology 1999; Front D, et al. Hodgkin disease: Prediction of outcome with Ga-67 scintigraphy after one cycle of chemotherapy. 210: 487-491
(8) Radiology 2001; Rahmouni A, et al. Mediastinal lymphoma: Quantitative changes in
gadolinium enhancement at MR imaging after treatment. 219: 621-628