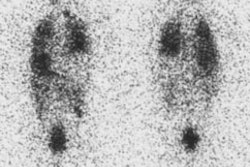
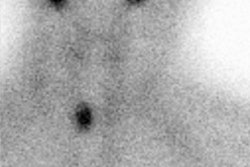
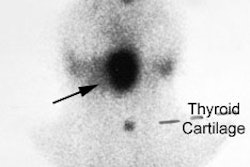
Radiotherapy In Non-Neoplastic Thyroid Disease:
Radiotherapy of thyroid disease uses the high energy beta particle emissions of 131I to treat hyperthyroidism due to Graves or Plummers disease. The beta particle has a maximum energy of 0.61 MeV, an average energy of 0.192 MeV, and a range in tissue of about 0.8 mm [10]. It is well recognized that solitary nodules and multinodular goiters are more radioresistant than Grave's disease, and that large glands are less sensitive than small ones. Generally, to achieve adequate treatment about 7000 rads should be delivered to the gland for diffuse toxic goiter, and between 15,000-30,000 rads for toxic nodular goiters (due to the non-homogeneous distribution of I-131 in these cases). A typical I-131 dose used for the treatment of Grave's disease delivers between 8 to 16 rads to the bone marrow. Antithyroid drugs should be discontinued for at least 48 hours prior to the therapy and preferably for 5 to 7 days as they may affect the uptake of radioactive iodine (see additional discussion below). If necessary, these agents can be resumed 7 to 10 days following treatment. Radioiodine has also been used in treating patients with large compressive goiters who are nonsurgical candidates. A decrease in thyroid volume by about 40% may occur following I-131 therapy [1,2]
Factors affecting treatment
- Iodine uptake by the gland
- Bulk of tissue to be destroyed
- Length of time radioactive iodine is retained in the gland: In euthyroid individuals the biologic half-time of radioiodine in the thyroid is about 90 days [13]. Hyperthyroid patients discharge radioiodine more rapidly with an average biologic half-time of only 30 days [13]. Most radioiodine leaves the thyroid as radiolabeled thyroxine (T4) and triiodothyronine (T3) which bind to serum proteins [13]. T4 and T3 have a prolonged residence time in the circulation and are responsible for up to 90% of the blood and whole-body radiation absorbed dose [13].
- Distribution within the tissue
- Radiosensitivity of thyroid cells: Hyperthyroidism secondary to a toxic nodular goiter [Plummer's disease] is particularly resistant to radioactive iodine treatment and frequently requires doses which are 2-3x's larger than those applicable in diffuse toxic goiter, and even with such doses, it is unlikely to induce hypothyroidism.
- Mode of delivery of I-131: A reduction in the percentage of radioiodine uptake has been reported when using capsules compared to liquid [9]. The variability in the bioavailability of radioiodide from a capsule can affect the outcome of a diagnostic study or therapy [9]. The presence of magnesium stearate in the capsules appears to affect the release of the iodine [9]. The use of a U.S. pharmacopeia dissolution test can be applied to the capsules as a quality assurance procedure [9].
- Drug interactions: Certain agents need to be discontinued prior to therapy [10]
- Renal function: Administered radioiodine that is not trapped in the thyroid is largely excreted via the kidneys [16]. The treatment dose generally dose not need to be adjusted for patients with end-stage renal disease [16]. For patients on dialysis, treatment should be performed as soon as possible following dialysis and there should be a delay in subsequent dialysis until maximum I-131 uptake has occurred [16]. Following treatment, dialysis should be performed in a private room, equipment and fluid disposed from the dialysis unit should be monitored by radiation safety personnel, and the radiation dose to the dialysis technician should be monitored [16].
| Medication | Withdrawl Period |
| Antithyroid medication (propylthiouracil, methimazole, carbimazole) and multivitamins | Propylthiouracil should be discontinued 3d
prior
to treatment. Methomazole and carbimazole for 5 days
[13,16], but up
to 2-3 weeks has been recommended [24], particularly
in patients with MNG to prevent
radioiodine uptake by normal thyroid tissue [15]. The agents
should not
be administered for 3-5 days following therapy to avoid
interference
with the treatment [15].
7 days for multivitamins |
| Thyroid hormone | 2 weeks for cytomel
4 to 6 weeks for synthyroid |
| Kelp, Lugol's solution, SSKI solution, and
iodine containing medications |
2 to 3 weeks |
| IV contrast | Over 1 month |
| Amiodarone | 3 to 6 months |
Contraindications to I-131 therapy
- Pregnancy: Radioiodine freely crosses the placenta. Additionally, activity in the maternal bladder causes significant fetal irradiation. The fetal thyroid extracts/concentrates iodine after the 11-12th week and the radiation will destroy the thyroid gland and result in severe hypothyroidism. A documented negative beta-HCG should be obtained, ideally within 24 hours prior to treatment, of any menstruating female [23].
- Breast feeding: Both iodine and pertechnetate are excreted in
breast milk. Breast feeding must be stopped following treatment
[23].
In order to minimize radiation dose to the breasts, therapy
should be
delayed until lactation ceases for breastfeeding women
(typically 4-6
weeks and preferably 3 months after breast feeding stops) [23].
- Severe thyrotoxicity: Patients should be pretreated (with beta-blockers) and anti-thyroid medications to avoid thyroid storm which can occur from sudden release of hormones following radiation destruction of the thyroid follicles.
- Radioiodine therapy should be considered with caution in
patients with Graves ophthalmopathy (especially in smokers)
because cell damage induced by the treatment causes relaease
into the circulation of thyroid antigens that enhance the
immunogenic stimulation leading to thyroid stimulating hormone
receptor antibody production that can promote de novo or
worsening Graves orbitopathy [24].
I-131 Therapy for hyperthyroidism/Graves disease
Technique
Graves disease is the most common cause of thyrotoxicosis [16].
I-131 is the treatment of choice for patients over the age of 30
years, or
those with medical
complications of their thyroid disease. It is now generally
accepted
that attempting to titrate the administered dose to render a
patient
euthyroid is not possible or wise and that the administered dose
should
be sufficient to cure the hyperthyroidism in a reasonable time
(less
than 6 months) [16]. The amount of I-131 to be administered is
approximately 100-200 uCi per
gram of thyroid [16]. By dividing this number by the measured
uptake of
the gland, an actual
dose can be determined. Unfortunately, estimates of the thyroid
weight
can be off by as
much as 20 to 50% (the normal thyroid is about 20 gms [16]). Many
centers treat patients with a standard dose of between 8 to 12 mCi
and perform no calculations. Larger fixed doses are generally more
reliable in controlling hyperthyroidism [16]. Patients should fast
for at least 4 hours prior to treatment to maximize GI absorption
[24].
Dose Determination: Dose= (Thyroid mass[gms] x 80-200 uCi/gm)/ Percent uptake
Despite the above efforts to determine the appropriate dose based upon gland size and radioiodine kinetics, the success of radioiodine therapy remains largely unpredictable [21]. This is because the current methods define whole thyroid as the target organ, although the biologic effects are primarily due to irradiation of thyroid follicular cells [21]. For the same thyroid mass and radioiodine uptake, patients with larger follicles would require larger therapeutic doses in order to achieve the same absorbed dose to the follicular cells [21]. In the future, it may be possible to incorporate this information into dose determinations [21].
Adjunctive use of Tapazole or PTU with I-131
Antithyroid drugs do not affect the iodine-trapping mechanism
(and
therefore radioactive iodine uptake) and do not interfere with the
initial binding of iodine to tyrosine residues on thyroglobulin
(organification) unless given in large doses [13]. The agents,
however,
do block formation of thyroxine and triiodothyronine even at low
doses
[13]. Concern over the rapid release of glandular hormone stores
after
I-131 treatment
provides the rationale for adjunctive therapy with antithyroid
drugs
before or after
therapy in order to deplete thyroid hormone [5].
Adjunctive use of antithyroid medication should be considered for older patients (over 50-60 years of age), very thyrotoxic patients, and patients with cardiac problems [16]. Some authors feel that the adjunctive use of antithyroid medications leads to a more rapid remission of hyperthyroidism than with I-131 alone and the combination may also reduce the risk of post-treatment thyroid storm and hypothyroidism. One other advantage of the use of antithyroid medications is to lower the peripheral body radiation dose- particularly in patients with rapid iodine turnover [13]. The disadvantage is that the concurrent use of these agents may decrease the effectiveness of I-131 and more I-131 is necessary for adequate treatment [4]. However, although there is some evidence that propylthiouracil increases the resistance of the thyroid to radiation, methimazole and carbimazole do not [16].
The drug is generally stopped at least 3 to 7 prior to treatment and may be restarted 2 to 3 days following I-131 administration [10] (the dose of I-131 should be increased by 25% if these agents are given within 14 days following treatment). Tapazole interferes with iodine metabolism (oxidation) and it also has an iodide diuretic effect.
Some authors contend that antithyroid drug therapy has little effect on the short term changes in thyroid hormone levels after radioiodine treatment compared to a control group [5]. Thus, pretreatment with thionomides may not reduce the risk of thyroid storm [5]. In patients at high risk for going into thyroid storm following I-131 treatment, an alternative treatment would be to administer pharmacologic doses of iodine as Lugol's solution or as a saturated solution of potassium iodide-SSKI. Iodine therapy can effectively inhibit the release of thyroid hormones although there can be an escape from this inhibitory effect after a few weeks.
Radiation safety:
When treating patients with I-131 it is important to counsel them
regarding radiation safety precautions. Arrangements need to be
established to ensure that no adult is exposed to more than 5 mSv
(500
mrem) as a result of the treatment [16]. Safety measures that
should be
followed include sleeping in a separate bedroom, using and washing
separate utensils, keeping ones toothbrush separate, and remaining
more
than than 2 m from family members for a few days [16].
Following therapy, a waiting time before conception of 6 months
is recommended in women and 4 months in men [24].
Results of treatment
No significant change in thyroid function can be expected for 3 to 6 weeks. The maximum effects of the therapy should occur between 3 and 4 months, but final results may not be known for 6 to 9 months. The cure rate is proportional to the dose deposited and runs from 50% (50 uCi/gm deposited) to 80% (190 uCi/gm deposited). There are three potential outcomes of therapy- patients may become euthyroid, remain hyperthyroid, or more commonly become hypothyroid. Between 5 to 15% (10%) of patients will remain hyperthyroid and will require a second treatment. Some authorities advise a second treatment after 3 months, but a delay of 6 months may be more appropriate as a proportion of patients respond later [16].
Some centers favor using a lower dose of I-131 in an attempt to render the patient euthyroid without the need for thyroid hormone replacement. However, this is associated with a higher treatment failure rate with patients often requiring a second or third treatment with I-131. Since hypothyroidism is almost an inevitable consequence of I-131 treatment for Graves' disease, larger doses of I-131 have been used with the purpose of ablating the thyroid and deliberately causing hypothyroidism [4]. Depending on the dose used for therapy, the incidence of hypothyroidism following treatment ranges from 50-80% during the first year and 1-3% per year thereafter. Following successful therapy, the thyroid gland may return to a normal size. It is generally recommended that pregnancy should be avoided for 6-12 months following treatment to allow for normalization of thyroid function [16,23].
Side Effects
In the first week after treatment, some patients may experience a
transient sore throat or mild
dysphagia.
Transient exacerbation of hyperthyroid symptoms:
Another potential, but rare complication of I-131 therapy for hyperthyroidism is temporary thyrotoxicosis (or thyroid storm {0.34% of patients [18]}) due to the sudden release of thyroid hormone from the gland as it is destroyed [6,23]. The period of greatest risk for thyroid storm is usually between 3 - 15 days after treatment (first 2 weeks [23]). This can be a potentially fatal complication. Patients who are at highest-risk include the elderly, those with severe thyrotoxicosis, severe weight loss, prolonged thyrotoxicosis, large glands or multinodular glands and markedly elevated levels of T3, or those with other underlying disease (such as coronary artery disease, congestive heart failure, or atrial fibrillation) [18]. There is no relationship between the dose of radioiodine and the risk of thyroid storm [18]. Providing a "cool down period" by pretreatment with antithyroid medications (PTU or Tapazole [thionamide]) and beta blockers for 6-12 weeks to deplete thyroid stores especially in high risk patients may help prevent excessive thyroid hormone release post-therapy [18]. Antithyroid medications should be discontinued 3-5 days prior to therapy, and then resumed 2-5 days after treatment (PTU also acts to decrease peripheral conversion of thyroxine to triiodothyronine) [18,23]. Beta blockers do not need to be discontinued prior to I-131 therapy [23]. However, the benefits of this approach has been questioned by Burch et al. and others [5,19]. Initiating oral iodides (i.e. SSKI) 2-3 days after I-131 treatment and continued for 5 days inhibits hormone release from the thyroid gland and may protect against thyroid storm [18]. Beta blockers should also be commonly employed as these agents help to reduce symptoms of thyrotoxicosis and also work to decrease peripheral conversion of thyroxine to triiodothyronine [18]. However, thyroid storm may still occur during beta-blocker therapy [18].
Gaves ophthalmopathy:
The data regarding radioiodine treatment and Gaves ophthalmopathy are controversial [20]. Ophthalmopathy may develop after therapy [10] and some studies have suggested an increase in the occular manifestations of Graves following I-131 treatment, compared to surgery or antithyroid medication [20]. Patients without clinical ophthalmopathy are less likely to be affected by RAI therapy; however, RAI therapy may exacerbate clinically evident ophthalmopathy and the use of adjunctive systemic corticosteroids following treatment may be indicated. However, the use of steroids (especially high doses) may result in a reduction in the effective I-131 half-life (although this does not seem to effect overall treatment efficacy)[22]. This may be related to steroid induced increased renal iodine clearance and decreased thyroid-stimulating antibodies that can result in reduced thyroid radioiodine uptake [22]. One author suggests that a 10-15% increase in the I-131 dose be considered for patients receiving high dose steroid therapy [22].
The reported rates of increased ophthalmic manifestations after I-131 treatment were 4-5% in all patients, and 23% in patients with pre-existing ophthalmopathy [20]. However, the clinical manifestations of the ophthamologic changes associated with I-131 treatment may not be significant [20]. Post-therapy hypothyroidism may aggravate the ophthalmopathy and should be prevented through the early institution of levothyroxine replacement therapy [4]. Smoking is associated with a higher risk for ophthalmopathy and worsening ophthalmopathy after I131 therapy and every effort should be made to encourage patients to stop [16,23,24]. Other factors associated with an increased risk for development or progression of ophthalmopathy include high pretreatment levels of serum triiodothyronine, post-therapy hypothyroidism, and high levels of TSH receptor antibodies [23,24].
Radiation thyroiditis:
Radiation thyroiditis is a very rare complication of I-131 therapy (1-5% of cases [23]) [16]. Symptoms are similar to subacute thyroiditis with pain radiating to the jaws or ears [16]. Treatment is with antiinflammatory medications or a short course of corticosteroids [16,23].
Recurrent laryngeal nerve palsy: Very uncommon [23].
Dysgeusia (altered or distorted sense of taste: Very uncommon
{23].
Neoplasm:
The risk of thyroid cancer following I-131 therapy for hyperthyroidism is actually reduced or not significantly different from the general population (this may be related to destruction of the thyroid gland) [8,23]. The risk of leukemia or other malignancy is no greater than in the general population. [7] Additionally, there has been no demonstrable harmful effect upon the health of progeny, fertility, or reproductive history [23]. No significant genetic effect has been demonstrated as well [23]. Nonetheless, women should be advised to refrain from becoming pregnant for at least 6 to 12 months following therapy. A 10 mCi dose of I-131 delivers a dose of about 2 rads to the ovaries. This dose can be reduced with hydration and frequent voiding. The typical dose to the blood is between 8 to 16 rads.
I-131 Therapy for the Autonomous Functioning Nodule
Clinical hyperthyroidism is uncommon in nodules smaller than 2.5 cm and if the patient is not toxic, radioiodine therapy is not indicated [4]. Toxic autonomously functioning adenomas are relatively radioresistant and effective treatment generally requires doses in excess of 20 mCi [4]. About 90% of autonomous nodules are ablated by a dose of 40,000 rads. Because the remainder of the thyroid is suppressed, the incidence of hypothyroidism following therapy is typically low [4] (reported in 4-44% of patients [17]). If the nodule is not ablated on the first dose, there is an increase dose requirement for re-treatment due to lower uptake by the nodule. In general, a good way to calculate the dose necessary to ablate a solitary toxic nodule is:
Dose I-131= 10 mCi/Uptake
Autonomous nodules can also be treated with percutaneous ethanol injection [3,11,17]. However, the treatment often requires multiple sessions and patients may experience severe pain in up to 21% of cases [17]. Other complications include recurrent laryngeal nerve dysfunction (typically transient) and abscess formation [17]. Given these risks, it is probably most useful for autonomously functioning nodules with a large fluid component to decrease the size of the nodule prior to effective I-131 therapy [15].
I-131 Therapy for Autonomously Functioning Multinodular Goiter (Plummer's Disease):
The gland is frequently very large, fairly radioresistant (due to the non-homogeneous distribution of I-131 within the gland), and iodine uptakes between 25 to 30% are not uncommon. Large doses are typically required (29.9 mCi). Treatment also helps to decrease the size of the thyroid gland [15]. In less than 1% of cases, immunogenic hyperthyroidism occurs following the treatment due to induction of TSH receptor autoantibodies [15].
I-131 Therapy for Non-toxic Nodular Goiter:
I-131 therapy is effective for the reduction of thyroid volume in patients with non-toxic multinodular goiter producing compressive symptoms [12]. Goiter size can decreased by about 40% after 1 year and 50-60% after 3 to 5 years [12]. The dose is approximately 100 uCi of I-131 per gram of thyroid tissue, corrected for RAIU at 24 hours [12]. Pre-treatment with rhTSH increases radioiodine uptake by more than 4-fold and allows the therapeutic dose to be reduced by about 50% without compromising treatment results [12,15]. Because thyroid hormone levels can increase by about 50% over baseline following rhTSH administration, beta blockers or calcium channel blcokers may be required to avoid thyroid-hormone mediated adverse effects [15].
REFERENCES:
(1) Am J Med 1988; Kay TW, et al. Treatment of non-toxic
multinodular
goiter with
radioactive iodine. 84: 19-22
(2) Ann Intern Med 1994; Huysmans DA, et al. Large, compressive goiters treated with radioiodine. 121: 757-762
(3) Radiology 2000; Tarantino L, et al. Percutaneous ethanol injection of large autonomous hyperfunctioning thyroid nodules. 214: 143-148
(4) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(5) Ann Intern Med 1994; Burch HB et al. Discontinuing antithyroid drug therapy before ablation with radioiodine in Graves' disease. 121:553-559
(6) Amer J of Medicine 1983; McDermott. Radioiodine-Induced Thyroid Storm Case Report and Literature Review. 75:353-359
(7) J Nucl Med 1993; Shapiro B. Optimization of radioiodine therapy of thyrotoxicosis: what have we learned after 50 years? 34: 1638-41
(8) J Nucl Med 2000; Angusti T, et al. Thyroid cancer prevalence after radioiodine treatment of hyperthyroidism. 41: 1006-1009
(9) J Nucl Med 2002; Yu MD, et al. The effect of formulation on reduced radioiodide thyroid uptake. 43: 56-60
(10) J Nucl Med 2002; Meier DA, et al. Procedure guideline for therapy of thyroid disease with 131iodine. 43: 856-861
(11) J Nucl Med 2003; Zingrillo M, et al. Percutaneous ethanol injection plus radioiodine versus radioiodine alone in the treatment of large toxic nodules. 44: 207-210
(12) J Nucl Med 2004; Nieuwlaat WA, et al. Dosimetry of radioiodine therapy in patients with nodular goiter after pretreatment with a single, low dose of recombinant human thyroid-stimulating hormone. 45: 626-633
(13) J Nucl Med 2004; Zanzonico PB, et al. Enhancement of radioiodine treatment of small-pool hyperthyroidism with antithyroid drugs: kinetics and dosimetry. 45: 2102-2108
(14) Thyroid 2006; Cooper DS, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. 16: 1-25
(15) Endocr Pract 2006; American association of clinical endocrinologists and associazione medici endocrinologi medical guidelines for the clinical practice for the diagnosis and managment of thyroid nodules. 12: 63-102
(16) J Nucl Med 2007; Iagaru A, McDougall R. Treatment of thyrotoxicosis. 48: 379-389
(17) AJR 2008; Tarantino L, et al. Percutaneous ethanol injection of hyperfunctioning thyroid nodules: long-term follow-up in 125 patients. 190: 800-808
(18) Am J Med 1983; McDermott MT, et al. Radioiodine-induced thyroid storm. Case report and literature review. 75: 353-359
(19) Ann Nucl Med 2006; Vijayakumar V, et al. Is it safe to treat hyperthyroid patients with I-131 without fear of thyroid storm? 20: 383-385
(20) J Nucl Med 2008; Sisson JC, et al. Radioiodine therapy and Graves' ophthalmopathy. 49: 923-930
(21) J Nucl Med 2008; Eterovic D, et al. Planning of 131I therapy for Graves disease based on the radiation dose to thyroid follicular cells. 49: 2026-2030
(22) J Nucl Med 2010; Hautzel H, et al. Qualitative and
quantitative
impact of protective glucocorticoid therapy on the effective 131I half-life in
radioiodine
therapy for Graves disease. 51: 1917-1922
(23) J Nucl Med 2012;
Siberstein EB, et al. The SNNMI practice
guideline for therapy of thyroid disease with 131I
3.0*.
53: 1633-1651
(24) J Nucl Med 2021; Mariani G, et al. The role of nuclear medicine in the clinical management of benign thyroid disorders, part 1: hyperthyroidism. 62: 304-312