Thallium Cardiac
Imaging
Clinical
Applications of Thallium Myocardial Stress Imaging:
1- Detection of coronary artery disease
Unfortunately, angiographically defined coronary artery disease does not necessarily correlate with necropsy data. Angiographically significant stenoses that equal or exceed 70% may possess sufficient coronary flow reserve or adequate collaterals. Conversely, luminal narrowings between 50-60% may be associate with a significant perfusion abnormality. Thallium imaging provides physiologic information regarding the impact a specific stenosis has on perfusion to the region subtended by the stenosis.
2- Evaluation of the extent and severity of coronary stenosis
In order to assess patient prognosis.
3- Assess myocardial viability:
Thallium is superior to Tc-Sestamibi in the evaluation of myocardial viability.
4- Assess outcome and efficacy of therapy
Thrombolytic therapy increases the likelihood of the patient having a patent infarct-related vessel and better preserved left ventricular function. Tc-Sestamibi may be the superior agent for imaging the extent of myocardial salvage following the institution of thrombolytic therapy.
5- Evaluation of patients who have an uninterpretable ECG stress test
In cases of left bundle branch block, digitalis therapy, or underlying ST segment abnormality.
Physical
Characteristics of Thallium:
Thallium is cyclotron produced from Tl-203 and therefore may contain
Pb-203
and Tl-202 impurities. It decays by electron capture to Mercury 201
with a
half-life of 73.1 hours. It emits gamma photons with energies of 135 keV (3%) and 167 keV
(10%). Due
to the low abundance of these gamma emissions, mercury characteristic
x-rays
are used for imaging. Mercury 201 emits x-rays between 68-80 keV (94.5%) and smaller amounts of gamma rays
with higher
energies. Because of the low energy of the imaged emissions, thallium
images
suffer from attenuation artifacts and can be of poor quality in obese
patients
[33].
The effective dose to the patient from a dose of 3.25 mCi of Tl201
is 16.8 mSv and the dose from a 4 mCi dose is 20.7 mSv [44].
Biodistribution of Thallium
Thallium is a potassium analog, but it is less readily cleared from the cell. About 60% of Tl-201 uptake is dependent upon a functioning sodium/potassium ATPase sarcolemmal membrane transport pump. There is also uptake via a separate non-energy dependent facilitated diffusion (or co-transport system). The cellular uptake of thallium is blocked by ouabain and digitalis because of inhibition of the sodium-potassium ATPase system. Flurosemide inhibits the co-transport system.
Thallium is rapidly cleared from the blood with a myocardial first pass extraction efficiency of about 85% under normal basal flow conditions [33]. Extraction of Tl-201 decreases at higher flow rates, however, extraction is still superior to sestamibi and tetrofosmin [33]. Tl-201 extraction is not affected by medications such as propanolol or insulin. Less than 5% of the administered dose remains in the blood pool at 15 minutes. Approximately 4% of the administered dose localizes to the myocardium which is about the percentage of cardiac output supplied to the coronary arteries. Peak myocardial activity occurs between 5 and 15 minutes after injection.
The excretion of thallium is predominantly renal and the kidneys are the critical organ (3-5 rad/mCi). There is a small percentage of GI excretion.
Redistribution
of Thallium:
The initial distribution of thallium in the myocardium is primarily a function of blood flow, however, Tl-201 does not remain fixed in myocardial cells following the initial extraction phase [33]. The agent is continuously exchanged with new Tl-201 from the systemic circulation- this process is referred to as redistribution [33]. Redistribution is the mechanism which permits thallium to detect myocardial ischemia and areas of hibernating (viable) myocardium. Redistribution reflects an equilibrium between tracer uptake and efflux. It is dependent upon blood flow and concentration gradients. It is also dependent upon the ability of the myocardial cells to retain thallium (ie: the capacity of the membrane Na-K ATPase in each myocyte to maintain high intracellular levels of thallium). Overall, thallium clearance is facilitated by good blood flow and high intracellular concentrations of the tracer, but also by cell injury which renders the cells unable to retain thallium secondary to membrane dysfunction. Normal thallium washout is about 30% of activity at 3 hours and about 35% at 4 hours following injection.
In general, thallium clears more slowly from regions supplied by stenotic vessels than from normal myocardial regions. Areas of significant hypoperfusion will have very slow clearance and may even accumulate thallium. This occurs because as thallium is cleared more rapidly from well perfused regions the intravascular concentration will increase resulting in a gradient from the vascular space to the low concentration (ischemic) regions (washout from normal areas and increased uptake in viable ischemic zones).
Thallium
Stress
Cardiac Imaging
Physiology
When atherosclerotic narrowing occurs in a coronary artery, the reduction in perfusion pressure causes the coronary arterioles to dilate in order to maintain resting coronary blood flow [15]. Blood flow through a diseased coronary artery at rest is not decreased until the stenosis exceeds 85-90% of the luminal diameter [13,15]. At this point, the coronary arterioles are fully dilated [15]. Thus, resting regional myocardial blood flow is usually homogeneous, even in the presence of significant coronary artery stenosis [13]. Therefore a resting perfusion defect on SPECT imaging indicates a critical coronary artery stenosis, a myocardial scar from infarction [41].
During exercise coronary vessels that contain smooth muscle in their walls will dilate (particularly the coronary arterioles), the coronary vascular resistance will decrease, and coronary blood flow will increase [15]. Coronary reserve is the ability to increase coronary blood flow in response to metabolic demand. A greater than 80% stenosis is required to cause resting ischemia [39], however, the coronary reserve is decreased when a coronary artery stenosis exceeds 40-50% (i.e.: despite maximal arteriolar dilatation, flow can no longer be increased to meet metabolic needs) [13,15,39]. Therefore, compared to rest imaging, an exercise exam is more likely to detect regions of ischemia. Adequate exercise stress is essential for the detection of ischemia. Inability to reach 85% of the maximal predicted heart rate during exercise may decrease the sensitivity of the exam [31].
Of course, when a coronary stenosis is present, the lumenal narrowing generated by the lesion is only partially responsible for the decreased coronary flow reserve [15]. Capillary derecruitment distal to the stenotic vessel occurs in an attempt to maintain a constant capillary hydrostatic pressure in the face of maximal arteriolar dilatation [15]. This derecruitment results in a decreased capillary surface area, which causes a reduction in the extraction of the radioisotope and the consequent perfusion defect seen on imaging [15]. The degree of capillary derecruitment distal to a stenosis during hyperemia is proportional to the severity of the stenosis [15].
To prevent medications from masking ischemia, beta-blockers should be withheld for 48 hours prior to the exam, and calcium channel blockers and long-acting nitrates should be held for 24 hours.
Technique:
The exam is performed using a 180 degree arc (1 projection every 6 degrees) from the RAO to LPO position for 30 uniformly spaced projection images [14]. This technique is used because myocardial counts in the remaining projections are overwhelmingly attenuated (by the spine) or scattered by the patients body and do not contribute much information to the images [14]. Images can be acquired in a circular, elliptical, or patient contoured orbit [14]. Non-circular orbits which follow the patient's body minimize the distance between the collimator and the heart which provides the best possible resolution and uniformity in each projection. However, artifacts may be caused by combining projection images with such different resolution characteristics in the reconstruction process without depth-dependent collimator response correction [14].
A. End points for the exercise stress test
1-
Attainment of predicted target heart rate
Double product = HR x Systolic BP. The double product is a reflection of myocardial oxygen demand. It should be greater than 20,000 or at least double from the patients resting value. In the normal heart, an increase in myocardial oxygen demand is accompanied by a parallel increase in flow so that a linear relationship exists between these two variables.
2-
Classic Angina
3- Over 3 mm of flat ST segment depression or elevation
* Region of ST segment depression may
not actually
correspond to the ischemic region.
* Region of ST elevation typically corresponds to region of ischemia.
4-
Drop in systolic BP greater than 20 mm Hg
Patients who demonstrate a decrease in blood pressure with exercise have an increased likelihood of CAD.
5-
Ventricular dysrhythmia
B. Anti-anginal medications
A normal thallium exam performed while the patient is on antianginal medication may underestimate the eventual need for revascularization. However, the risk of cardiac death or non-fatal MI in patients with a normal thallium exam is very low, and the clinical outcome is not affected by use of antianginal medications (beta blockers, calcium antagonists) during the exam or by the level of stress achieved [1,2]. Nonetheless, the sensitivity of thallium in detecting coronary artery disease is lower with submaximal exercise, as up to 50% of defects occur at heart rates in excess of 85% of the maximally predicted heart rate [3].
C.
Reinjection
The reinjection thallium stress exam is potentially a 3 part examination. At maximal stress (one minute prior to completion of exercise) 3 mCi of thallium is injected and post-stress images obtained (image acquisition should begin within 10 minutes of completion of exercise). Three to four hours later, redistribution images are performed. If these images are normal, or if they demonstrate clear cut ischemia, the exam is complete. If the studies demonstrate a fixed perfusion defect, an additional 1 mCi to 1.5 mCi of thallium is injected and another set of images obtained 15 to 30 minutes following reinjection to permit clearance of background activity.
Reinjection is performed because routine redistribution imaging will overestimate the prevalence and severity of irreversible myocardial damage (i.e.: many stress-induced perfusion defects in ischemic myocardium do not return to normal on routine redistribution imaging). Between 40-50% of fixed defects on 4-hour redistribution images demonstrate improved or normal regional thallium activity after thallium reinjection. Late imaging (at 24 hours post injection) allows more time for thallium to accumulate into low flow regions and will also permit detection of additional ischemic regions. Although 24 hour redistribution images will detect additional areas of ischemia, up to 40% of defects which remain fixed at 24 hours will also demonstrate improved tracer accumulation following reinjection at 24 hours [4]. The majority of regions (80-87%) with evidence of viability following reinjection will demonstrate improvement in both thallium uptake and resting ventricular function after revascularization.
It is believed that reinjection improves the concentration gradient
of
thallium from the blood into areas of ischemia, thus producing improved
detection of ischemic regions. In contrast, only 0 to 18% of regions
with
persistent defects following reinjection demonstrated functional
improvement
after revascularization. Delayed imaging at 24 hours after reinjection
identifies additional reversible segments in only 6% of patients, and
is not
routinely performed [7]. In the evaluation of fixed defects a separate
day
stress-redistribution and rest-redistribution exam will detect a
greater number
of reversible segments compared to a stress-redistribution-reinjection
protocol
(36% vs. 27%) [8]. The typical total-body effective dose from a Tl-201
stress
and reinjection exam (3.0 mCi + 1.0 mCi) is between 1.8 to 2.5 rem
(18 to 25.1 mSv) [37,38,43].
A
stress redistribution thallium exam using a 3.5 mCi dose results in a
radiation exposure of about 15.3 mSv [43].
Early reinjection (ie: immediately following completion of stress imaging) may not be an accurate predictor of eventual reversibility and probably overestimates fixed defects in regions of actual ischemia.
Thallium SPECT with nitrates and reinjection may improve detection of ischemic but viable myocardium in comparison to reinjection alone (without redistribution image acquisition prior to reinjection). Up to 25% of defects which appear fixed on reinjection images, are reversible on nitrate/reinjection images. A long acting nitrate such as isosorbide dinitrate (half-life 4-5 hours) can be administered following completion of stress images and its effects should persist throughout the duration of the exam. Nitrates have been shown to increase the regional blood flow to ischemic myocardial regions [9,10].
|
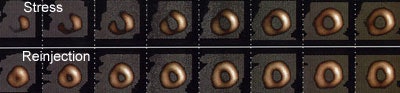
Thallium Stress-Reinjection Examination: The short axis images below are from a stress-reinjection thallium exam. There is severe, ischemia involving the anterior wall, septum, inferior wall, and apex. The left ventricular chamber is slightly more prominent on the stress exam (Click image to enlarge and see entire study) |
|
|
Differential uptake is a drawback of reinjection imaging. It refers to the mistaken identification of an ischemic region as a fixed defect. Following reinjection, regions supplied by normal vessels will have proportionally more thallium deposited in them than regions supplied by stenotic vessels. As a result of this "differential uptake", between 2 to 10% of areas which may have appeared reversible on routine redistribution images, can appear as fixed defects if only reinjection images are performed. Up to 25% of segments which appear as fixed defects on quantitative analysis of reinjection images, may actually be ischemic [8,11,12]. These ischemic regions can be identified by obtaining routine rest images prior to reinjection, or by obtaining 24 hour delayed images in patients with fixed reinjection defects.
Sensitivity/Specificity
of
Thallium Exam
Exercise ECG: Sensitivity 70%, Specificity 80%. The sensitivity of the exercise ECG tends to be lower in female patients. It is believed that estrogen has a digoxin-like effect that can produce varying changes in the ST segment.
Planar thallium: Sensitivity 83%, Specificity 90%
Planar thallium with quantitative analysis: Sensitivity 90%, Specificity 90%, Normalcy 95%
SPECT Thallium (stenoses greater than 50%): Sensitivity 90%, Specificity 75-88% (Low specificity may be related to referral bias and technical artifacts), Normalcy: 89%. Normalcy is a better indicator of the true specificity of the exam. It represents true negative exams in patients with a low likelihood of disease (less than 5% pretest likelihood for coronary artery disease) [32]. The sensitivity of SPECT thallium tends to be lower in female patients, while specificity remains comparable to that of men.
Confounding
Factors:
Eating between stress and rest imaging reduces blood thallium levels due to gut activity and preferentially increases clearance from ischemic regions (in other words, it diminishes thallium redistribution into ischemic myocardial regions). Thus, actual ischemic regions may appear to be fixed defects.
The myocardial clearance rate of thallium appears to be slower after dipyridamole than exercise possibly because of a lower myocardial to blood pool gradient, despite an initial higher myocardial activity after pharmacologic stress. This lower gradient is most likely related to higher blood pool activity caused by high liver clearance and the splanchnic bed acting as a reservoir, releasing the isotope slowly into the blood [5,6]. Despite these references, I am not sure why activity released from the muscles after exercise stress does not produce the same effect. Neither of these articles addresses this issue.
Extravasation of the dose at the injection site may also reduce the examinations sensitivity for detecting ischemia by altering both thallium delivery and the washout gradient.
Normal
Variants:
Mild decreased activity is commonly seen in association with:
Apical
thinning
Normal apical thinning subtends an arc of less than 30 degrees. The defect is usually symmetric about the apex and there is no redistribution.
Basal
segments
Due to diaphragmatic attenuation. Attenuation correction programs can be used to decrease this problem, however, they often overcorrect and can decrease the sensitivity of the exam to identify true inferior/basal ischemia [16].
Upper
septal area
The membranous septum typically has little or no activity compared to the muscular septum.
Right
ventricular activity
Under normal conditions the right ventricle has approximately 60% less myocardial mass and corresponding less total myocardial blood flow than the left ventricle [36]. RV activity can be seen during exercise, but should be noted only faintly at rest. RV activity on rest images is associated with right ventricular pressure overload (>30mmHg), volume overload, or both. In patients with pulmonary arterial hypertension, development of right ventricular enlargement and dysfunction (cor pulmonale) is associated with poor survival. When pulmonary artery pressure is raised the right ventricle is susceptible to ischemia due to limited coronary reserve for the hypertrophied ventricle. Patients with pulmonary hypertension have greater right ventricular thallium uptake after dipyridamole stress which increases with increasing myocardial mass. Patients with the most RV uptake have the most significant impairment of RV systolic function, independent of ischemia [17].
Another finding associated with RV hypertrophy is loss of the interventricular septum's normal convexity into the right ventricle. Isolated right ventricular volume overload produces dilatation of the RV with maintenance of septal convexity toward the right ventricle. It can occur secondary to an ASD, VSD, or tricuspid regurge.
Increased
Pulmonary Activity
Pulmonary activity is an indicator of left ventricular end-diastolic pressure. The amount of pulmonary activity is dependent upon the heart rate obtained. If an individual failed to achieve adequate cardiac stress one would expect a greater amount of pulmonary activity. In general, in an adequately stressed patient, pulmonary activity should be less than 50% of cardiac activity (Heart to lung activity ratio greater than 2:1). Increased lung activity is seen in association with:
1. Marked exercise induced left ventricular dysfunction. Increased pulmonary activity is felt to reflect pulmonary edema secondary to transient global LV dysfunction and elevated left ventricular end diastolic pressure [40]. It may be the only evidence of 3-vessel disease (global ischemia).
2. Pulmonary venous hypertension (Mitral valve disease).
Increased pulmonary activity is associated with a markedly increased risk of future cardiac events. An abnormal heart to lung ratio is a poor prognostic indicator and is perhaps the most important prognostic indicator for the likelihood of future cardiac events [18]. The positive predictive value of increased lung uptake for subsequent cardiac event is approximately 70% (the negative predictive value is 86%).
As with exercise thallium imaging, increased pulmonary activity on dipyridamole stress exams is also a marker of functionally more significant coronary artery disease [34]. Myocardial to background ratios are comparable in both exercise and dipyridamole imaging, most likely due to a relatively greater myocardial uptake of the tracer with pharmacologic stress. Although dipyridamole should not induce ischemia, perhaps there is vasodilator induced subendocardial hypoperfusion as a result of a "steal phenomenon" which results in LV dysfunction and increased LV filling pressures [20,34].
Dilatation
of the left ventricle with stress
Transient left ventricular (LV) dilatation refers to an apparent increase in LV size at stress, relative to the rest images [30]. Scintigraphic evidence of left ventricular dilatation with stress is an indirect indicator of extensive coronary artery disease (i.e.: the ischemia may be more extensive than indicated by perfusion defects alone). There is a strong correlation (sensitivity 60%, specificity 95%) between LV stress dilatation and the presence of multivessel CAD (often with critical stenoses [greater than 90%]). Stress induced left ventricular dilatation is a poor prognostic indicator [21]. Cardiac death and non-fatal myocardial infarction rates are significantly higher in patients with transient dilatation [30]. Transient dilatation can also be seen following pharmacologic stress and it is also associated with more severe coronary artery disease and a worse prognosis [21,27,35]. The finding most likely represents a diffuse reduction in subendocardial flow reserve.
Actual ventricular dilatation is less likely to be noted on images performed with technetium imaging agents due to the delay prior to imaging. If it is seen, it most likely represents the presence of diffuse subendocardial ischemia and not transient ischemic chamber dilatation due to the lack of redistribution of the agent (i.e.: technetium agents provide a snap-shot of perfusion at the time of injection) [30]. The decreased subendocardial uptake with stress produces the visual impression of a larger chamber and thinner walls compared to the rest images [30].
Stress dilatation can also be identified with dual-isotope (thallium/sestamibi) imaging. Using an automated algorithm for endocardial border detection a left ventricular endocardial volume stress- rest ratio of greater than 1.22 has been shown to be a sensitive (71-77%) and specific (92-95%) indicator of severe and extensive coronary artery disease [23]. The ratio is determined by automated quantitative software and this measured ratio is more accurate than visual analysis [23].
Other causes of transient dilatation of the left ventricle with stress in the absence of coronary artery disease include: 1- Severe hypertensive heart disease due to subendocardial ischemia associated with delayed diastolic left ventricular relaxation, decreased capillary density in the hypertrophied myocardium, and elevated end-diastolic pressures [22]; and 2- Dilated cardiomyopathy due to limited coronary flow reserve [22].
Reverse
redistribution
Reverse redistributions occurs when there is worsening of a perfusion defect or the appearance of a new perfusion defect on redistribution thallium imaging [18,28]. Reverse redistribution does not indicate ischemia [18]. Regions of myocardium which demonstrate reverse redistribution have activity which appears similar to adjacent regions on the initial scan which indicates a non-critical stenosis in the supplying artery or adequate collateral circulation. These areas, however, demonstrate a more rapid clearance of thallium than normal which is felt to be related to: 1- an inability of the myocytes in these regions to retain thallium (ie: less than a full complement of normal functioning myocytes); and 2- initial thallium uptake within non-viable myocardium and the interstitial space. Reverse redistribution can be seen with both stress-redistribution and rest-redistribution imaging (see discussion in Rest-Redistribution imaging section).
This pattern has been most commonly observed in patients who have had an acute myocardial infarction and have undergone treatment with coronary thrombolysis or another form of revascularization [18]. In this setting, reverse-redistribution is frequently associated with patency of the infarct related artery, non-transmural infarction, and relatively normal wall motion in the affected region [24]. In the post-revascularization period there is a mixture of scar and stunned myocardium which probably results in the poor retention of tracer. Thus, early after reperfusion, reverse redistribution may be seen as evidence of infarct-related artery patency and has a better prognosis than the classic reversible defect. It has also been described soon after angioplasty or bypass surgery, again in the presence of a patent graft or supplying artery. In patients with known chronic CAD, this finding may represent the presence of scar within a segment containing viable myocardial elements- whether normal, stunned, or hibernating. [25]
In the general population, reverse redistribution has been found to be associated with coronary artery disease. Frequently such segments are supplied by occluded epicardial vessels (50% of cases), with good collateral circulation (seen in 60%). It is postulated that heterogeneous washout from these segments results in reverse redistribution [29]. Segments demonstrating reverse redistribution are also noted to have abnormal wall motion (in up to 33% of segments [28]).
Reverse redistribution has also been described in coronary artery spasm [42], and a variety of cardiomyopathies including sarcoidosis and Chagas' disease. It is important to remember, however, that in patients with a low pretest likelihood of disease, this finding can represent normal variability in the clearance of thallium or an imaging artifact.
Preliminary observations from the Thrombolysis and Myocardial Infarction trial suggest that reverse-redistribution is associated with a higher incidence of future cardiac events independent of the presence of ischemia. Such events (defined as unstable angina or acute MI) occurred in 30% of patients- particularly those with severe reverse-redistribution. About three-quarters of segments with reverse-redistribution are viable by PET FDG imaging and viable segments generally demonstrate normal wall motion on the MUGA exam. Segments with wall motion abnormalities have over a 50% chance of representing scar [19].
Reverse redistribution can also be the result of artifacts such as differences in breast position between stress and redistribution imaging [29]. Use of color scales can also result in apparent reverse redistribution. After exercise each of the 256 levels of the color scale samples a relatively large range of pixel values because the myocardial tracer uptake is high and the range between the highest and lowest pixel values is wide [29]. After redistribution, the range between the highest and the lowest pixel value is narrower. Therefore, 2 pixels coding for two close values, displayed in the same color on exercise, may be displayed with 2 different colors at rest, inducing false reverse redistribution [29]. This is particularly obvious when bowel activity is included in the reconstructed volume and is higher than myocardial uptake [29].
In the setting of revascularization following an acute myocardial infarction, reverse-redistribution can also be seen on technetium agent imaging if delayed images are performed [26]. Mitochondrial membrane potential alterations likely occur following a period of ischemia and revascularization [26]. This alteration contributes to a decreased capacity to retain technetium perfusion agents [26]. None-the-less, delayed images are typically not acquired when using these agents.
REFERENCES:
(1) J Nucl Med 1993; Brown KA, Rowan M. Impact of antianginal medications, peak heart rate and stress level on the prognostic value of a normal exercise myocardial perfusion imaging study. 34: 1467-1471
(2) J Nucl Med 1994; Beller GA. Myocardial perfusion imaging with thallium-201. 35: 674-680
(3) J Nucl Med 1993; Ignaszewski AP, et al. Safety and clinical utility of combined intravenous dipyridamole/symptom-limited exercise stress test with thallium-201 imaging in patients with known or suspected coronary artery disease. 34: 2053-61
(4) J Am Coll Cardiol 1991; Kayden DS, et al. Thallium-201 for assessment of myocardial viability: quantitative comparison of 24-hour redistribution imaging with imaging after reinjection at rest. 18:1480-86
(5) J Nucl Med 1993; Schulman DS, et al. Right ventricular thallium-201 kinetics in pulmonary hypertension: relation to right ventricular size and function. 34: 1695-700
(6) J Nucl Med 1994; Lee J, et al. Biokinetics of thallium-201 in normal subjects: comparison between adenosine, dipyridamole, dobutamine and exercise. 35: 535-41
(7) Circulation 1991; Dilsizian V, et al. Thallium reinjection after stress-redistribution imaging. Does 24-hour delayed imaging after reinjection enhance detection of viable myocardium? 83: 1247-54
(8) J Nucl Med 1995; Inglese E, et al. Assessment of myocardial viability after thallium-201 reinjection or rest-redistribution imaging: a multicenter study. The Italian Group of Nuclear Cardiology. 36: 555-63
(9) J Nucl Med 1993; He ZX, et al. Nitrates improve detection of ischemic but viable myocardium by thallium-201 reinjection SPECT. 34: 1472-77
(10) Circulation 1992; 86(Supp): I-109
(11) Circulation 1992; Dilsizian V, Bonow RO. Differential uptake and apparent 201Tl washout after thallium reinjection. Options regarding early redistribution imaging before reinjection or late redistribution imaging after reinjection. 85: 1032-1038
(12) Dilsizian, Circulation 1993; Dilsizian V, Bonow RO. Current diagnostic techniques of assessing myocardial viability in patients with hibernating and stunned myocardium. 87: 1-20
(13) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(14) J Nucl Med 2001; Germano G. Technical aspects of myocardial SPECT imaging. 42: 1499-1507
(15) J Nucl Cardiol 2001; Kaul S. The role of capillaries in determining coronary blood flow reserve: implications for stress-induced reversible perfusion defects. 8: 694-700
(16) J Nucl Med 2001; Harel F, et al. Clinical impact of combination of scatter, attenuation correction, and depth-dependent resolution recovery for 201Tl studies. 42: 1451-56
(17) J Nucl Med 1993; Schulman DS, et al. Right ventricular thallium-201 kinetics in pulmonary hypertension: relation to right ventricular size and function. 34: 1695-700
(18) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(19) J Nucl Med 1995; Soufer R, et al. Relationship between reverse redistribution on planar thallium scintigraphy and regional myocardial viability: a correlative PET study.36: 180-87
(20) Am J Cardiol 1990; Villanueva FS, et al. Prevalence and correlates of increased lung/heart ratio of thallium-201 during dipyridamole stress imaging for suspected coronary artery disease. 66: 1324-28
(21) Am.J.Cardiol 1990; Lette J, et al. Transient left ventricular cavitary dilation during dipyridamole-thallium imaging as an indicator of severe coronary artery disease. 66:1163-70
(22) AJR 2000; Robinson VJB, et al. Causes of transient dilatation of the left ventricle during myocardial perfusion imaging. 174: 1349-1352
(23) J Am Coll Cardiol 1996; Mazzanti M, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilatation of the left ventricle in dual-isotope myocardial perfusion SPECT. 27: 1612-1620
(24) J Nucl Med 1995; Ohte N, et al. Clinical significance of reverse redistribution on 24-hour delayed imaging of exercise thallium-201 myocardial SPECT: comparison with myocardial fluorine-18-FDG-PET imaging and left ventricular wall motion.
(25) J Nucl Med 1993; Liu P, Burns RJ.
Easy come,
easy go: time to pause and put thallium reverse redistribution in
perspective.
34: 1692-94
36: 86-92
(26) J Nucl Med 2001; Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. 42: 1351-1358
(27) J Nucl Cardiol 2002; Hansen CL, et al. Comparison of pulmonary uptake with transient cavity dilatation after dipyridamole Tl-201 perfusion imaging. 9: 47-51
(28) J Nucl Med 2002; Roelants VA, et al. Reverse redistribution on exercise-redistribution 201Tl SPECT in chronic ischemic dysfunction: predictive of functional outcome after revascularization? 43: 621-627
(29) J Nucl Med 2002; Faraggi M. The continuing story of 201Tl reverse redistribution: reverse redistribution is still alive, but is the myocardium still viable? 43: 628-631
(30) J Nucl Cardiol 2002; McLaughlin MG, Danias PG. Transient ischemic dilatation: a powerful diagnostic and prognostic finding of stress myocardial perfusion imaging. 9: 663-667
(31) J Nucl Cardiol 2003; De Lorenzo A, et al. Use of atropine in patients with submaximal heart rate during exercise myocardial perfusion SPECT. 10: 51-55
(32) J Nucl Cardiol 2003; Beller GA. Clinical value of myocardial perfusion imaging in coronary artery disease. 10: 529-542
(33) J Nucl Cardiol 2004; Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. 11: 71-86
(34) J Nucl Cardiol 2004; Goland S, et al. Dipyridamole-induced abnormal Tl-201 lung uptake in patients with normal myocardial perfusion: a marker of increased left ventricular filling pressures. 11: 305-311
(35) J Nucl Card 2005; Abidov A, Berman DS. Transient ischemic dilatation associate with poststress myocardial stunning of the left ventricle in vasodilator stress myocardial perfusion SPECT: true marker of severe ischemia? 12: 258-260
(36) J Nucl Cardiol 2005; Wackers FJ. On the bright right side. 12: 378-380
(37) J Nucl Cardiol 2006; Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. 13: 19-23
(38) Radiology 2008; Earls JP, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. 246: 742-753
(39) J Nucl Med 2008; Vesely MR, Dilsizian V. Nuclear cardiac stress testing in the era of molecular medicine. 49: 399-413
(40) J Nucl Med 2008; Patel D, et al. Diastolic filling parameters derived from myocardial perfusion imaging can predict left ventricular end-diastolic pressure at subsequent cardiac catheterization. 49: 746-751
(41) J Nucl Cardiol 2008; Travin MI. The oft neglected rest study. 15: 739-742
(42) J Nucl Cardiol
2011; Xiang D, et al. Investigation of the mechanism of reverse
redistribution
in thallium-201 myocardial perfusion scintigraphy
in
patients with suspicion for coronary artery spasm. 18: 314-323
(43) J Nucl Cardiol 2012; DePuey EG, et al. Patient-centered
imaging. 19: 185-215
(44) J Nucl Cardiol 2012; Duvall WL, et al. The prognosis of a
normal Tl-201 stress-only SPECT MPI study. 19: 914-921