CHICAGO - An automated breast ultrasound system (ABUS) can provide reproducible results in localizing, characterizing, and sizing breast masses, according to a presentation Wednesday at the 2010 RSNA meeting.
A research team from Seoul National University Hospital in Korea found a high level of image quality in pairs of ABUS scans, as well as strong reproducibility in lesion detection and characterization.
In conjunction with mammography, breast ultrasound is a valuable tool for evaluating and characterizing breast lesions. And while performing screening ultrasound in high-risk patients can increase cancer detection, ultrasound does suffer from problems such as extreme operator dependency, a high degree of user error, and poor reproducibility, said presenter Jung Min Chang, MD.
ABUS are designed to solve these problems and can theoretically provide reproducibility, an important benefit for the technology to be used as a screening method and to enhance reliability of follow-up data, she said. As a result, the researchers sought to retrospectively evaluate the reproducibility of data acquisition of an ABUS for mass detection, localization, and characterization.
Between October 2007 and March 2008, ABUS scans were performed twice within an interval of less than two months in 84 patients who were scheduled for biopsy or surgical excision. The researchers excluded 47 patients who underwent biopsy before the second ABUS scan, 11 patients who had multiple breast lesions, and two patients whose data were lost.
As a result, 24 patients (mean age, 49 years) with 33 breast lesions were included in the study. Of the 33 lesions, 24 were breast cancers and nine were benign lesions. The cancers had a mean size of 1.6 cm, with a range of 0.6-5.0 cm, while the benign lesions had a mean size of 0.9 cm, with a range of 0.6-1.2 cm.
Using an ABUS from U-Systems of San Jose, CA, two trained technicians performed whole-breast ultrasound exams on the patients with a mean interval of 1.3 days. A standardized acquisition technique was used, with a lateral and medial scan performed for each breast.
Scans were obtained before biopsy or surgery. Three-dimensional coronal volumetric data, axial data, and reconstructed sagittal data were reviewed, Chang said.
For image analysis, two breast radiologists assessed image quality and lesion visibility through a consensus reading. Overall image quality was rated on a five-point scale (1: unacceptable; 2: poor; 3: fair; 4: good; and 5: excellent) based on visualization of anatomic details and artifacts.
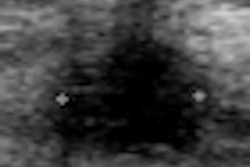
Lesion visibility was assessed as either depictable or nondepictable, Chang said. To determine reproducibility, the researchers placed two serial exams side by side and recorded automated documented lesion location, including clock face, distance from nipple, lesion depth, and lesion size.
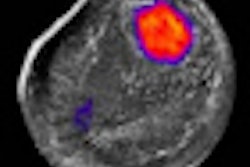
Next, lesion similarity was evaluated, including recording of BI-RADS features and classifying morphological similarity of the lesions as identical, similar (not identical but having same BI-RADS ultrasound lexicon), or different (lesions having at least one different BI-RADS feature that may lead to different final assessment), she said.
The mean image quality was 4.6, with a range of two to five. Three cases were rated with poor image quality, due to focal defects caused by skin detachment, she said.
All lesions included in the scanned areas were depictable with information comparable to handheld ultrasound systems, according to Chang. However, two lesions were not included in one of the paired 3D datasets due to their peripheral location.
Evaluation of overall lesion-based reproducibility showed nearly perfect agreement between the two serial exams with the exception of lesion depth. "Lesion depth showed low correlation due to different scanning pressure," she said.
As for morphological similarity, 22 cancers and 7 benign lesions were assessed as identical or similar. Two benign lesions were assessed differently, Chang said.
"For better reproducibility, correct placement of the receptor without missing the periphery and adjustment of scanning pressure is still essential," she said.
By Erik L. Ridley
AuntMinnie.com staff writer
December 1, 2010
Related Reading
Automated breast US proves to be sensitive screening tool in dense breasts, November 29, 2006
Auto breast US handily spots lesions in early study, January 13, 2006
Copyright © 2010 AuntMinnie.com