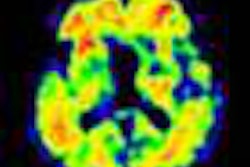
Piramal Imaging has received U.S. Food and Drug Administration (FDA) approval for its Neuraceq PET imaging agent.
Neuraceq is indicated for PET imaging of the brain to estimate beta-amyloid plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive decline, Piramal said.
The approval comes four weeks after Piramal receiving marketing authorization from the European Commission. Piramal has partnered with IBA Molecular to manufacture and distribute Neuraceq.
The FDA approval of Neuraceq is based on safety data from 872 patients who participated in global clinical trials, as well as three other studies that examined images from adults with a range of cognitive function, including 205 end-of-life patients who had agreed to participate in a postmortem brain donation program.
Images were analyzed from 82 subjects with postmortem confirmation of the presence or absence of beta-amyloid neuritic plaques, according to Piramal. Correlation of the visual PET interpretation with histopathology in these 82 brains demonstrated that Neuraceq accurately detects moderate to frequent beta-amyloid plaques in the brain.