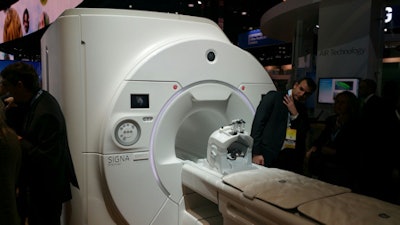
GE Healthcare said its 3-tesla wide-bore MRI system, Signa Premier, is pending 510(k) clearance by the U.S. Food and Drug Administration (FDA).
Signa Premier was designed for research, particularly in neurology and oncology, GE said. Approximately 100 subjects have been scanned to date at the University of Wisconsin in Madison, GE said.
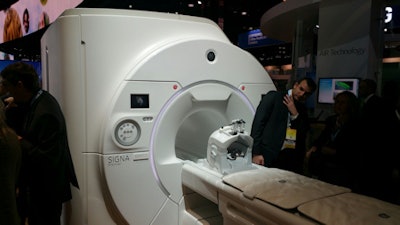 Signa Premier from GE Healthcare.
Signa Premier from GE Healthcare.The Signa Premier platform includes a short-bore, high-homogeneity magnet; new gradients; a new digital radiofrequency (RF) transmit-and-receive architecture; and new applications including cloud-based analytics, GE said.


















