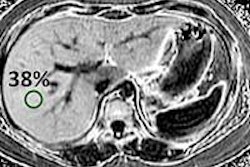
Medical device firm Nevro has received the European CE Mark for expanded MRI-conditioned labeling of its Senza implantable spinal cord stimulation (SCS) system.
Senza can now be marketed in Europe and Australia for use with 1.5-tesla and 3-tesla MRI scanning of the head and extremities under specified conditions, according to the vendor. The new labeling, which adds support for 3-tesla MRI, includes all generations of the Senza SCS system dating back to 2010. It also applies to all geographies where Nevro is currently selling the system.