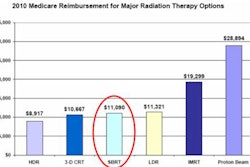
How does a busy radiation oncology department adopt and implement a new treatment technology with which it has no prior experience? Through careful planning, effective teamwork, and ample time allocated for training and development of a rigorous quality assurance program.
There's nothing revolutionary about these components for success. They're the core building blocks of new technology adoption. But as international concern escalates about the utilization of increasingly complex radiotherapy equipment, a primer explaining the practical aspects of adding a helical tomotherapy device for intensity-modulated and image-guided radiotherapy without creating havoc in a cancer treatment center is timely indeed.
The U.K. experience
Radiation oncologists, medical physicists, radiographers and radiation therapists, and administrative staff of the Oncology Centre at Addenbrooke's Hospital in Cambridge described their experiences in an article published in the May issue of Clinical Oncology (2010, Vol. 22:4, pp. 294-312). This Cambridge University NHS Foundation Trust hospital was the first in the U.K. to add a helical tomotherapy modality integrating image-guided radiation therapy (IGRT) and intensity-modulated radiation therapy (IMRT) to its program.
Tasks that the oncology center undertook included planning for adoption, installation, and validation; identifying differences from standard practices of radiation therapy; establishing protocols for patient selection, imaging, and quality assurance; measuring workflow to improve efficiency; and modifying schedules to enable staff to receive comprehensive training. Also, because this was the only unit of its kind, contingency backup plans to provide for uninterrupted patient treatment needed to be developed.
The system (Hi-Art, TomoTherapy, Madison, WI) was installed in August 2007, under the direction of a clinical project leader. The department spent the next two months developing quality assurance programs and commissioning the device.
Because no official recommendations on routine quality assurance were available, the Oncology Centre's medical physicists needed to develop them. They also developed patient-specific quality control protocols to be used before the first treatment of every new patient. In addition, staff from the National Physical Laboratory in London made baseline measurements to obtain measures of absolute dose, because this had not been done previously in the U.K.
Training
Lead author Dr. Neil Burnet, a radiation oncologist and honorary consultant oncologist at the Oncology Centre, recommended that access by all clinical staff to a tomotherapy planning station and treatment unit, or spending time in a training center or at another active tomotherapy site, before the start of clinical treatments would be a major advantage and facilitate a faster start-up.
In fact, Burnet and colleagues recommended that a single national center be established in the U.K. to provide standardized training. They also suggested that training software simulating imaging and treatment of virtual patients be developed to provide the necessary hands-on experience.
Medical physicists and dosimetrists received training from the vendor during the installation and commissioning period. Prior to basic training on the device, a CT anatomy training program was developed for the radiographers/radiation therapists, and they used a phantom to practice imaging and image matching.
In addition to taking formal vendor training courses, radiation oncologists spent time working with the equipment at already-established sites in Europe. Tomotherapy planning was also practiced on dummy datasets before clinical implementation. This provided better medical understanding of the strengths and weaknesses of the system -- knowledge that the authors stated was essential.
Patient selection and scheduling
From the outset, the center had more patients who could benefit from receiving treatment from the unit than it had capacity. The treatment team also wanted to implement a manageable ramp-up and workload that would allow all members of the multidisciplinary team to have ample time to thoroughly learn to use the system. Not overscheduling to accommodate for a learning curve proved to be critically important for the efficient adoption of the system.
Criteria for patient selection were determined by those who would benefit from IGRT because of target movement, such as patients with prostate cancer. Patients who would benefit from IMRT included those with complex target volume shapes or adjacent critical normal tissue structures, and where conventional plans required field junctions. These predominantly were patients with head and neck cancers.
All patients had to be able to remain on the treatment table for a 20-minute period without experiencing undue pain, breathlessness, or claustrophobia. Excessively large or obese patients could not be accommodated.
From the outset, the center established a database to record acceptance and rejection data, patient toxicity, local control, and survival outcomes.
Two patients were selected for treatment starting October 31, 2007. In December, seven patients were treated per day on average, and in January the daily workload was 10 patients. During the first 12 months, 2.6 patients began treatment each week, with an average of 11.2 new starts each month. The total number of fractions delivered per day was capped at 22 for the first 21 months, and was subsequently increased to 24 when workflow efficiencies permitted. Utilization averaged 80% capacity, with an average of 17.5 patients treated during an eight-hour day.
Ultimately, 114 patients were accepted for treatment in the first 12 months. Of the first 100, 3,127 fractions were delivered. The median dose was 66 Gy, with a range of 8 to 74 Gy, and the median number of fractions was 33, with a range of three to 39 fractions.
Workflow
The center recorded treatment times in detail from the outset, recording the data manually using a clock. A custom software program was developed to quickly replace this cumbersome method. Times from patient entry into the treatment room through exit were recorded, as well as positioning time, imaging time, matching time, and beam-on time. This data enabled workflow to be measured and also were used for scheduling. In the first year, the median in-room time was 24.2 minutes, ranging from 22.7 to 26.7 minutes depending on the type of tumor.
The room was staffed with two radiographers/radiation therapists and one assistant practitioner to maximize throughput. A software upgrade in August 2008 increased computer communication speed, increasing capacity to 22 fractions per day. A couch upgrade in April 2009 decreased median in-room time to 18.4 minutes.
Cancer treatment centers whose therapists are experienced with volumetric CT imaging may have faster patient throughput statistics from the output, according to the authors. They suggested that the efficiency gains were more significant because their radiographers/radiation therapists were new to CT imaging and needed to acquire hands-on experience.
Treatment planning and patient positioning
Treatment planning consists of CT imaging data acquisition, outlining regions of interest, and planning treatment using the tomotherapy planning station. Axial CT images are acquired with a 3-mm slice thickness and spacing, with the exception of cranial images, which are acquired at 1 mm.
Because the treatment couch is included in the imaging dataset, patients must be positioned relatively high in the scanner bore to have 10 cm of image data below the surface of the couch. The field-of-view also cannot be restricted, because otherwise the couch data will be removed. The planning computer will remove the image of the couch and replace it with a representation of the treatment machine couch, which it includes in the final dose plan.
Before the acquired images are transferred to the tomography planning station, targets need to be outlined, using standard target volume structures with a conventional outlining tool. The planning station calculates dose-volume constraints for all target and normal tissue structures. Dose constraints can be added or particular structures blocked from radiation beam entry. The user also defines the machine characteristics of field width, modulation factor, and pitch.
Burnet reported that a modulation factor of 2 or 3 is usual, with the latter used for more complex, intricate shapes. Users specify a maximum value for the modulation factor, and the planning system selects the actual value. Subtle improvements in high dose conformation can be made by increasing the modulation factor, but the tradeoff is that the treatment will take longer to deliver. This is also true when the pitch, the degree of overlap between successive helices, is reduced.
The Oncology Centre prescribes a patient target volume dose as the median dose, using an added condition to achieve coverage of 99% of the target volume with 95% isodose.
When treatments begin, patients have daily CT fanbeam images using the shortest length possible within the region of the patient target volume. Treatment radiographers determine whether a thickness of 2, 4, or 6 mm should be utilized, based on the spatial resolution of the image needed balanced against the dose from imaging, and the speed required to perform the scan.
Daily target positioning discrepancies are measured by comparing the daily CT image with the original CT planning scan. An automated match measures discrepancies in all translational and rotational directions to determine if there is any gross error in the patient's position. When gross errors are detected beyond 6° of freedom, the patient is repositioned on the couch, rescanned, and matched again. This happened only nine times with the first 100 patients, or less than 0.3%.
The automated matching process is repeated with 4° of freedom, measuring discrepancies in all translational directions plus roll. Corrections are made as necessary. The center records all positional discrepancies in an in-house database, and the data are used to calculate population-specific margins and to identify systematic errors and trends for individual patients. This data collection revealed some interesting issues with the treatment couch.
One of the problems that the authors identified was treatment couch "sag," a factor dependent upon patient size, weight, and setup position. When patents were driven into the bore in the anterior-posterior axis, the authors discovered that the carbon-fiber couch top moves clear of the pedestal and flexes. In the superior-inferior direction, the authors determined that the systematic discrepancy in the direction of the head of the couch was larger than would result from vertical sag alone. They speculated that the couch top is dragged away from the pedestal and slightly into the bore against the longitudinal drive mechanism.
The authors emphasized the importance of careful patient setup to prevent positioning errors that cannot be automatically compensated for by the system, and use of immobilization devices if appropriate. The radiographers/radiation therapists are expected to position the patient as accurately as possible in order for the repositioning data to represent the initial positioning accuracy. The data have particular relevance if alternative IGRT strategies are considered, the authors explained.
Backup plans
The center developed a backup plan strategy over time by trial and error. Initially, because no second Hi-Art unit was available anywhere else in the U.K., the staff felt that backup plans were needed for treatment delivery on conventional linear accelerators. However, this significantly affected treatment planning workload, because it effectively required the preparation of two separate treatment plans for each patient.
After determining that backup plans were used in only eight patients of the first 30, and for five of them only one time, backup plans were prepared only for patients where interruptions in treatment are known to adversely affect outcome. This proved to be applicable only for patients with head and neck cancers. In several cases, these patients were offered hyperfractionation treatments of two fractions on the next day rather than using the backup treatment plan for conventionally delivered radiotherapy.
The authors reported that during the first year, the longest breakdown lasted 2.5 days and affected 17 patients, of whom one had not yet begun treatment. They made the decision to stop preparing backup plans altogether, determining that it was more time-efficient to prepare them only when needed. With the addition of a second Hi-Art unit, and in view of the reliability of the system, backup plans no longer have the same level of importance as they did when implementation strategies were being planned.
Update: May 2010
Implementing helical tomotherapy for IGRT and IMRT treatment was so successful that Addenbrooke's Oncology Centre acquired a second Hi-Art unit in November 2009. Commissioning by the medical physicists was scheduled for six weeks but only took five. If the commissioning schedule had not included taking extensive sets of beam data with a water tank for a research project, it may have been completed in four weeks, Burnet told AuntMinnie.com in an e-mail.
With the addition of the second unit, and having maximized workflow efficiency, the Oncology Centre can deliver up to 70 fractions per day, at a capacity of 30 on the system operating for eight hours, and 40 for the one operating 9.5 hours.
Cancer patients are referred for treatment from throughout the U.K. Between April 2010 and March 2010, a total of 5,393 patients were treated: 3,305, or 61%, for curative intent and the remaining 39% for palliative care.
Careful planning, which included ample training and a sensible ramp-up to accommodate learning curves, enabled a very busy cancer treatment clinic to adopt, learn to use, and become proficient with optimizing workflow of a complex new technology. In the authors' opinions, the experience that Addenbrooke's Hospital had should encourage the adoption of new radiation therapy technologies by similar cancer treatment centers.
By Cynthia E. Keen
AuntMinnie.com staff writer
May 18, 2010
Related Reading
FDA schedules meeting on RT error reduction, May 10, 2010
Societies to host meeting on radiation safety, April 16, 2010
Rad therapy groups support FDA review, April 13, 2010
TomoTherapy hits Hi-Art milestone, April 12, 2010
FDA turns attention to radiation therapy devices, April 8, 2010
Copyright © 2010 AuntMinnie.com