Radiation therapy equipment developer RefleXion Medical has appointed a new vice president of clinical, regulatory, and quality assurance.
Kathy O'Shaughnessy will be responsible for leading RefleXion's clinical, regulatory, and quality organizations as the company seeks U.S. Food and Drug Administration (FDA) and CE Mark clearance for its biology-guided radiotherapy (BgRT) system. She had previously served at R2 Technology, Xoft, Allux Medical, and Miramar Labs.
The company also announced that it has hired a new senior director of product, Calvin Huntzinger. Huntzinger will be responsible for overseeing RefleXion's clinical partnerships with academic cancer centers. He joins the firm after more than 25 years at Varian Medical Systems.
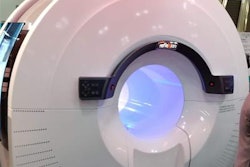
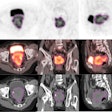
RefleXion's biology-guided radiotherapy system uses both CT and PET imaging data to guide personalized radiotherapy, the company said.