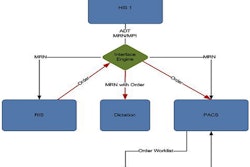
Carestream Health has received clearance from the U.S. Food and Drug Administration (FDA) for a module that enables lesion management tools to be used on its Vue PACS workstation software.
The tools are designed to improve the assessment of oncology patients by providing semiautomatic tracking and segmentation of lesions from modality scanners and PACS networks from other vendors. Each measurement generates an anatomical bookmark within the exam, according to Carestream, which radiologists can easily navigate and diagnose.
Radiologists can also use the tools to generate oncology imaging reports that comply with industry standards such as Response Evaluation Criteria in Solid Tumors (RECIST). Such standardized reporting allows for clearer communication and collaboration between radiologists, oncologists, and referring physicians, according to the company.
The tools are available as an optional native PACS module for the Vue software and can eliminate the requirement for separate dedicated workstations. Carestream will highlight the software at the upcoming RSNA 2012 meeting.