MINNEAPOLIS - How will the imaging community respond to the challenge of quantitative imaging for treatment response? That was the question that multiple speakers set out to answer in a symposium at last week's American Association of Physicists in Medicine (AAPM) meeting. MRI, PET, and SPECT are all being thoroughly scrutinized in order to meet the increasing demand for noninvasive imaging biomarkers that can not only customize treatment, but also track treatment response.
A major issue that the medical physicists who are developing these tools must contend with is standards for imaging quality, safety, and outcome goals. Creating uniform measurements from the different instruments and analytical tools -- not only in standalone institutions but also in multicenter trials -- make the standardization of cross-platform data particularly complex.
Functional MR
There’s been an explosion of MR imaging biomarkers in pre-clinical studies, as well as phase I/II clinical trials over the past few years, according to Edward Jackson, Ph.D., from the MD Anderson Cancer Center at the University of Texas in Houston. These MR biomarkers include measures obtained from dynamic contrast-enhanced MRI (DCE-MRI), dynamic susceptibility change MRI (DSC-MRI), diffusion-weighted MRI, blood-oxygen level dependent (BOLD) MRI, diffusion tensor imaging (DTI) and MR spectroscopy (MRS).
Both DCE-MRI and DSC-MRI have demonstrated an ability to evaluate the effects of antiangiogenic/antivascular therapy on a microvascular system, Jackson said. Diffusion MR is better suited for assessing cell volume and density changes, while DTI can spot white matter changes. BOLD is suitable for pinpointing changes in the oxy- and deoxyhemoglobin ratio. Finally, biochemical shifts can be measured with MRS.
But there are several unresolved barriers to development of imaging standards, Jackson said, including the lack of standardized acquisition pulse sequences and analysis techniques. Reproducibility, validation studies, and whether these MR studies will be reimbursed are also questions currently without answers.
But the growing push for noninvasive imaging biomarkers for the early assessment of treatment response will likely lead to biomarkers for both morphology and function, Jackson said.
"As with other modalities that provide means of early response to therapy, there is a tremendous opportunity for functional MR techniques to contribute to drug development, treatment assessment, and to selection of patient-specific therapies," he said.
PET and SPECT
The metabolic information provided by PET and SPECT has great potential for diagnosing and staging disease, customizing treatment doses for a particular patient, and tracking a patient's response to treatment, according to Janet Saffer, Ph.D., who is with the radiology department at the University of Pennsylvania in Philadelphia. But quantifying the information in these images remains challenging. Saffer described three key variable groupings in PET and SPECT that challenge standardization: patient-related, instrument-related, and operator-related.
Patient-related factors include body habitus, which affects attenuation and scatter, and physiology, which affects radioactive tracer distribution. Other patient-related factors are flexibility, ability to hold still, dosing, and, with fluorodeoxyglucose (FDG) imaging, the blood glucose level and uptake time.
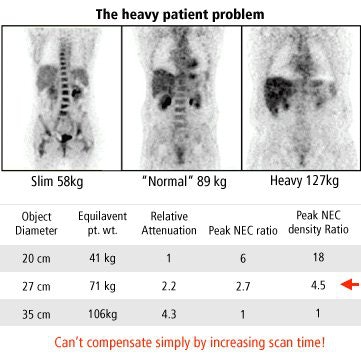 |
| Graphic courtesy of Janet Saffer, Ph.D., University of Pennsylvania, Philadelphia. |
Instrument-related variables are spatial and energy resolution of the scanner, sensitivity (with higher sensitivity resulting in lower Poisson noise), how PET data is acquired (2D versus 3D, with 2D reducing scatter but also sensitivity), and the intensity and scatter correction method used.
Saffer also compared time-of-flight (TOF) and non-TOF (used to measure the time that it takes for a particle to reach the detector at a known distance) images from a single patient to illustrate how TOF of an injected tracer affects image resolution.
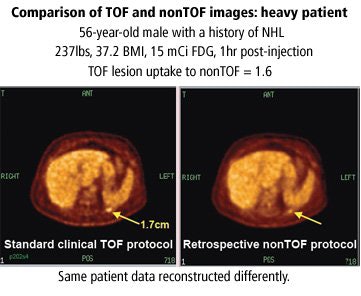 |
| Graphic courtesy of Janet Saffer, Ph.D., University of Pennsylvania, Philadelphia, and Philips Medical Systems, Andover, MA. |
Operator-related factors that affect quantification are how the images are to be acquired, and reconstruction protocols (e.g. arms up/down, imaging time per bed position), and instrument and image quality control, calibrations, and image analysis and interpretation.
Another issue that needs to be addressed is trying to merge data from multicenter trials when the information was obtained on different imaging platforms. A member of American College of Radiology Imaging Network (ACRIN), Saffer was on the team responsible for credentialing 100 participating PET programs. Participant groups needed to meet performance standards in resolution, sensitivity, scatter fraction, and count rate.
"We found an 11% failure rate in the first 53 applications," Saffer said, adding that use of multiple analysis tools make it difficult to specify a uniform measurement that all participants can accomplish. In its search for options, ACRIN is considering a proposed initiative to address standardization through open-source tools, she added.
FDG-PET
Andrei Pugachev, Ph.D., a medical radiation physicist at Sunnybrook Health Science Center in Toronto, discussed the use of PET biomarkers in radiation oncology, and the validation of FDG-PET imaging as a tool to gauge treatment response. He also described efforts to help more radiation oncologists incorporate FDG-PET into treatment planning.
Investigations into whether FDG-PET can reliably predict early therapeutic response have involved measuring FDG values before and after therapy. Prior to therapy, high FDG uptake values would signify its entrapment in the cancer cell. Following therapy, lower FDG uptake would signify decayed cancer cells. Pugachev said proposed approaches for graphically depicting tumors using FDG-PET should -- but haven’t -- taken into account the:
- Complexity of the tumor boundary (assuming one exists)
- Highly heterogeneous morphology of a lesion
- Variability of intratumoral FDG uptake, even in homogeneous animal models
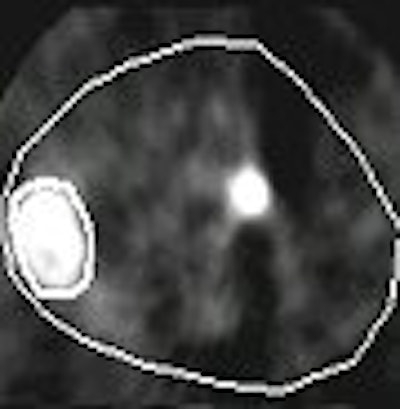
In one small-animal study, Pugachev compared tumor uptake results using FDG versus FMISO (fluoromisonidazole, an agent used for detecting hypoxic tumor). Some researchers believe FMISO can help predict tumor reoccurrence (International Journal of Radiology, Biology, Physics, April 1, 2005, Vol. 61:5, pp. 1493-1502).
 |
FMISO-PET (above) and FDG-PET (below) of human pharyngeal squamous cell carcinoma (FaDu tumor) in rat image twice with microPET. Images courtesy of Andrei Pugachev, Ph.D.
 |
While the tools for tumor segmentation are being developed through improved tissue penetration and tracer tracking, "we still can't differentiate between bound and unbound cells," Pugachev said.
Pugachev suggested that FDG uptake alone isn't enough to demonstrate therapeutic response. He said that more radiation oncologists might incorporate the use of PET imaging into their treatment planning once higher standards for tracer validation are established.
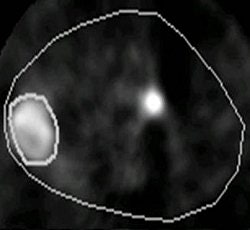 |
Animal PET imaging done with Cu-Diacetyl bis (N-4-methylthiosemicarbazone) or CuATSM in R3327-AT tumor. Above, at two hours postimaging and below, at 16 hours postimaging. It has been shown that tumor blood flow, which in some respect is complimentary to hypoxia, is also a negative prognostic indicator. Images courtesy of Andrei Pugachev, Ph.D.
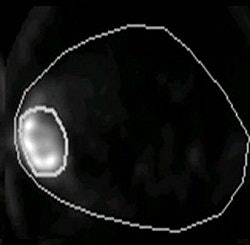 |
Animal tumor modeling and patient tumor specimens should be studied to show "that the tracer is in fact binding to the desired target," he explained. Another recommendation Pugachev had for fellow medical radiation physicists undertaking studies in therapy response was to adopt a probability approach rather than an either-or approach to treatment planning.
"For example, gradual change of FDG uptake from low level in normal tissue to high level in the lesion has to be interpreted as a gradual change in probability of finding a tumor cell, rather than used to randomly assign location of a step-like boundary," he said
Moving forward
Now is the opportune time to pursue imaging biomarkers and measurement standards, said Laurence Clarke of the National Cancer Institute in Bethesda, MD, although he added that imaging has been "late to the game" in terms of standardization.
Measurement uncertainty rests largely with the large volume of hardware and software sources in the imaging field, Clarke said. The wide range of modalities has added to the increasingly burdensome size and expense of clinical drug trials. He cited a recent National Institute of Standards and Technology (NIST) workshop in which the participants noted that the costs of bringing new treatments before the FDA are soon to exceed $1 billion. He called for the development of standardized methods to stimulate development of improved imaging methods and software tools.
Clarke predicted that medical physicists engaged in the development of imaging systems, and related standards, that may be used in trials for drug and therapy response should expect to rely heavily on industry support due to ongoing flat government funding for science and medicine.
One potential opportunity for funding may be the Biomarkers Consortium, a "public-private biomedical research partnership" launched by the NIH Foundation, Clarke said. A half dozen other federal agencies have similar (and largely unfunded) goals to set standards and study imaging as a biomarker for therapeutic response.
Standards for safety, quality, and quantitative measures will need support and guidance from many entities, central among them the medical physicists working in both public and private ventures who developed the instrumentation and technology used in diagnostic radiology, Clarke concluded.
By Kathlyn StoneAuntMinnie.com contributing writer
July 31, 2007
Related Reading
Studies link Alzheimer's to beta-amyloid and potential for new ligand, July 26, 2007
Possible new biomarker for breast cancer identified, April 16, 2007
Philips, BG Medicine align on molecular medicine R&D, August 11, 2006
Copyright © 2007 AuntMinnie.com