J Thorac Imaging 1999 Jan;14(1):37-50Pulmonary disease in the immunocompromised child.
Pennington DJ, Lonergan GJ, Benya EC
Department of Radiology, Indiana University Medical Center, Indianapolis 46202-5200, USA.
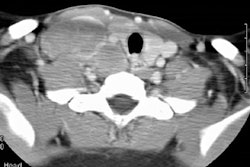
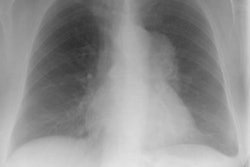
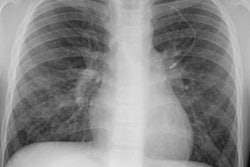
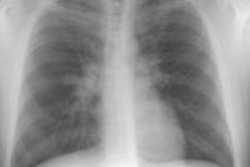
Immune deficiency states in children may be related to primary immunodeficiency syndromes or secondary disorders of the immune system. The secondary immunodeficiencies in children include human immunodeficiency virus-associated acquired immunodeficiency, as well as immunosuppression secondary to antineoplastic chemotherapeutic agents, bone marrow transplantation, and drugs given to prevent transplant rejection. This article discusses the common primary and secondary immunocompromised states of childhood, with emphasis on their attendant infectious, lymphoproliferative, and neoplastic complications.
Publication Types:- Review
- Review, tutorial
PMID: 9894952, UI: 99110243
Lymph > LIP
Latest in Lymphoproliferative
Lymph > Nodular lymphoid hyperplasia
February 12, 2014
Lymph > Pulmonary lymphangiomatosis
January 3, 2010
Lymph > Pulmonary lymphangiectasis
January 3, 2010
Lymph > General
April 2, 2002