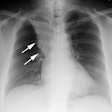
Lung Transplantation:
Complications discussed below:
- Reimplantation response (Reperfusion edema)
- Graft dysfunction
- Hyperacute rejection
- Acute rejection
- Bronchiolitis obliterans / Chronic rejection
- Airway complications: 1- Bronchial Dehiscence 2- Stricture
- Vascular
- Infection 1- Bacterial/Viral pneumonia 2. Opportunistic organisms
- Post-transplant lymphoproliferative disorders and other malignancies
- OKT3 Toxicity
- Hyperinflation of the native lung
- Recurrence of primary disease
Clinical:
Lung transplantation continues to gain acceptance for the treatment of end-stage lung disease- particularly emphysema and idiopathic pulmonary fibrosis. Lung transplantation is also used in patients with sarcoidosis, eosinophilic granuloma, extrinsic allergic alveolitis, lymphangioleimyomatosis (LAM), pulmonary hypertension, cystic fibrosis, and severe bronchiectasis [1]. Sarcoid, lymphangioleimyomatosis, diffuse panbronchitis, Langerhans' cell granulomatosis (eosinophilic granuloma) [15,16], pulmonary alveolar proteinosis, and giant cell interstitial pneumonitis have been reported to recur in the transplanted lung [1,26]. Sarcoid is the most common recurrent disorder- seen in up to 35% of cases [26]. LAM recurs in about 10% of patients [26].
Single transplantation is preferred as bilateral transplant does not significantly alter patient survival, the initial morbidity and mortality is slightly less than that of bilateral transplant, and the lungs from one donor can be used to benefit two patients [1,2]. Double lung transplant is used for patients with cystic fibrosis since a single transplanted lung would be susceptible to spread of infection from the native lung [3] and in patients with severe pulmonary hypertension to minimize hemodynamic instability [1]. Patients treated with double lung transplant tolerate bronchiolitis obliterans syndrome better than patients with single transplant [1].
In patients who undergo single lung transplant for emphysema, the implantation of a small donor lung can result in allograft compression by the hyperexpanded native emphysematous lung [33]. For donor selection, a 10-25% difference in size between the donor and the recipient lungs is acceptable [33]. Only blood group typing is performed [12]. The recipient must have no history of a recent malignancy and should be taking less than 20 mg of prednisone per day. Patients under the age of 65 years are candidates for single transplant, and patients under the age of 55 years for bilateral transplant. Patients with multisystem diseases are considered to be poor candidates for lung transplant. Patients with cystic fibrosis and pulmonary infection with pan-antibiotic resistant organisms (especially Pseudomonas cepacia) have high rates of reinfection after transplant and increased mortality. All patients being evaluated for lung transplant undergo CT imaging of the chest and all non-calcified nodules need to be evaluated to exclude malignancy [1].
Survival for patients undergoing single lung transplantation is approximately 70-90% at 1 year, 50% at 3 years, 35% at 10 years, and 25% at 15 years. The most common cause of mortality is infection in the first 6 months and chronic graft dysfunction thereafter [33]. Prognosis for survival is best in patients with obstructive pulmonary disease as their reason for transplant [1].
X-ray:
Following transplantation for fibrotic lung disease there is typically a shift of the mediastinal structures to the remaining native lung due to its low compliance compared to the transplanted lung. In transplants for emphysema, a moderate shift away from the overly compliant native lung may be observed. As a general rule of thumb: Opacities seen during the first week are usually due to reimplantation response (reperfusion edema). Persistent opacities beyond one week suggest infection or acute rejection. Infection during the first post-transplant month is usually bacterial, while opportunistic infections start to become more common after that time. Nodular opacities can be due to infection, post-transplant lymphoproliferative disorder, or recent transbronchial biopsy.
Solid and cavitary lung nodules can be seen 1 to 2 weeks following transbronchial biopsy in 30-35% of patients. On CT these nodules typically have a ground glass halo surrounding them and slowly resolve over 4 to 32 weeks. Nearly all patients develop a unilateral pleural effusion following the procedure and the majority of these are loculated. Effusions are common due to impaired fluid clearance through the lymphatics of the visceral pleura which slowly reconstitute and become functional within a month following transplant [12]. The majority of effusions will slowly resolve over weeks to months and variable residual pleural thickening or calcification may be observed. About 30% of effusions (especially loculated lesions) will require interventional drainage either due to enlargement or to exclude empyema. Only about 10% of effusions are found to be infected, however, this may be related to the common use of broad spectrum antibiotics in these patients [4]. Pleural effusions are also associated with acute rejection which is common 2 to 6 weeks following transplant [12].
Complications of lung transplantation include:
Reimplantation Response (Reperfusion Edema):
The reimplantation response is felt to be a form of non-cardiogenic pulmonary edema [33]. The pathogenesis of reperfusion edema is not clearly understood and it is probably multifactoral [29]. Causes include surgical trauma, allograft ischemia with increased vascular permeability, denervation, decreased surfactant production, and lymphatic interruption [29]. The condition occurs in more than 97% of transplanted lungs [1]. Reimplantation response is a diagnosis of exclusion- left ventricular failure, transplant rejection, fluid overload, and infection must all be excluded. The response almost always begins by the first post-transplantation day (typically within the first few hours [29], although other authors indicate that it typically occurs more than 24 hours following transplantation [33]), and is always present by day 3. It frequently progresses over the first few days, but peaks by day 4-5 and then begins to resolve. Another etiology such as infection or rejection should be considered for any new process beginning after this time. Most patients have normal findings or only minimal residual abnormality by 10 days after transplant [1,9]. However, severe edema can persist in up to 15% of all lung transplant patients and can result in primary graft failure [29].
Radiographically it appears as perihilar and/or basal ground-glass or air space opacities [33]. Reticular interstitial disease in the same distribution is also commonly seen. In double lung transplant patients, the disorder is asymmetric in 50% of cases. The response is noted to gradually change from air-space to reticular interstitial disease over the first few weeks and then gradually clear. The time to complete clearance can vary from 2 weeks to 6 months [3].
Graft Dysfunction:
Early graft dysfunction occurs within the first 24 hours after transplant. It occurs in under 10% of cases and is characterized histologically by diffuse alveolar damage. The dysfunction is usually the result of severe donor lung ischemia, donor lung injury, or vascular anastomotic stenosis [1].
Hyperacute Rejection:
Hyperacute rejection occurs immediately after transplant and in cases of an immunoglobulin G donor-specific HLA antibody positive crossmatch [10]. It results in acute diffuse alveolar damage [10]. Radiographically there is diffuse homogeneous infiltration of the entire allograft [33].
Acute Rejection:
Most patients will develop at least one episode of rejection within the first 3 weeks following transplantation, typically in the first 5 to 10 days. Most lung transplant recipients will experience two or three episodes of acute rejection within the first 3 months following surgery [28]. Patients with rejection develop can develop dyspnea, fever, leukocytosis, and a widened A-a gradient, however, patients with mild rejection can be asymptomatic. Pulmonary function testing may show a decrease in forced expiratory volume in one second and vital capacity [20]. Transbronchial biopsy is usually performed to establish the diagnosis (sensitivity 72-94%). There is often a dramatic response within 24 hours to treatment with corticosteroids and increased immunosuppression. The significance of acute rejection lies in the possibility that repeated episodes may lead to chronic rejection (bronchiolitis obliterans) [28].
Pathologically acute rejection manifests initially as a perivascular lymphocytic infiltrate. With progression this infiltrate becomes more widespread and extends into the alveolar septa and subsequently into the alveoli [20].
In about half the cases of rejection the chest radiograph will be normal. If seen, the findings are often non-specific such as new, worsening, or persistent perihilar and basal reticular interstitial disease (septal lines) and/or consolidations beyond 5 to 10 days after transplant. The findings can be asymmetric in double transplant patients. New or increasing pleural effusions without a concomitant increase in cardiac size, increase in vascular pedicle width, or vascular redistribution may also be an indication of acute rejection (sensitivity 68%, specificity 90% [12]).
Findings on HRCT are non-specific and include ground-glass opacities (often with a basal distribution [33]), peribronchial cuffing, septal thickening, ill-defined nodules, consolidations, new or increased pleural effusion, and reduced volume of the graft [28,33]. Ground glass opacities can be found in up to 65% of cases- they tend to be patchy and localized in mild rejection, and more widespread in severe rejection [5]. If findings are present, rejection can be confirmed by their rapid clearing typically within 48 hours of steroid therapy [1]. Overall, HRCT has a very low sensitivity (about 35-65%) and specificity (about 73%) for the diagnosis of acute rejection [28,33].
On Tc-MAA perfusion imaging, acute rejection was associated with deceased transplant perfusion characterized by a shift of flow to the native lung (normally, following transplant there is preferential flow to the transplanted organ). This shift reversed following therapy with steroids [3,12].
Bronchiolitis Obliterans/Chronic Rejection: (Bronchiolitis obliterans syndrome)
"Bronchiolitis obliterans syndrome" (BOS) is a major long term complication following lung transplantation [32]. BOS describes the progressive deterioration of graft function after lung transplantation not explained by acute rejection, infection, or problems of the bronchial anastamosis [18,22]. It is primarily a disease of the small airways, but late in the disease, large airway involvement can also be seen. Patients usually present with complaints of progressive dyspnea, a decline in exercise tolerance, and a non-productive cough. The clinical diagnosis of BOS is established when pulmonary function test results indicate obstructive small airway disease in the absence of other causes, 3 or more months after transplantation [14]. A decrease in FEV1 may occur before clinical symptoms appear [18]. A sustained decline in FEV1 to a level 80% or less of the best post-operative value is considered a reliable predictor of bronchiolitis oblietrans syndrome [14,18,25]. One of the earliest findings is a decline in flow rates at low lung volume; such as forced expiratory flow at 25 to 75% of vital capacity less than 70% of predicted. However, in patients with a single lung transplant for emphysema, the FEF 25%-75% values are usually abnormal regardless of graft function, owning to abnormalities in the remaining native lung [22]. In patients with a single lung transplant, a decrease in FEF 25%-75% of more than 20% from baseline may be a more reliable indicator of BOS [22].
To establish a diagnosis of bronchiolitis obliterans requires a lung biopsy and histologic assessment. In transplant patients, bronchiolitis obliterans (BO) probably represents chronic rejection and develops in about 40-50% of long term survivors of heart-lung transplantation and in 35-80% of lung transplant patients. Most patients develop BO after 6 months to within the first two years after surgery, but the condition can occur at any time [18,35]. Post-transplant patients that develop BO have a decreased survival compared to those that do not develop this condition [18]. The mortality from BO is between 25-50%. There is no specific treatment once BO occurs- retransplantation is the ultimate therapeutic option for progressive disease. BAL has not been shown to be useful for the detection of BO [18]. Transbronchial biopsy (sensitivity 15-78% [18]) may fail to establish the diagnosis due to the patchy distribution of the disorder [32]. Open lung biopsy may be required to confirm the diagnosis [18].
The major risk factor for the development of BO is multiple
episodes of acute rejection. The greater the number of episodes of
rejection in the first 6 months post-transplant (more than 3), and
the more severe those episodes, the more likely a patient will be
to develop BO [18]. Early diagnosis and treatment of episodes of
acute rejection is essential if BO is to be avoided [18]. CMV
infection may play a role in the development of BO and antiviral
prophylaxis with ganciclovir may help to prevent or delay the
onset of BO [18]. Another risk factor is human leukocyte antigen
mismatching [35].
Bronchiolitis oblietrans is often very patchy in its distribution. Chest radiographs in these patients are either normal or demonstrate non-specific findings such as hyperinflation, or attenuation of vascular markings. HRCT findings in BO include evidence of bronchiectasis (up to 80% of cases) and peripheral areas of lucency or mosaic perfusion which are related to distal airway obstruction with air-trapping (best identified on expiratory images), local hypoxia, and a decrease in vascular markings probably secondary to reflex vasoconstriction. [1,8,18] The presence of air trapping on expiratory HRCT images has a sensitivity of 74-91% and a specificity of 67-94% for the diagnosis of BO [22]. Other findings include bronchial wall thickening, interlobular septal thickening, peribronchovascular infiltrates, and nodular or linear branching opacities [33]. Unfortunately, HRCT is of limited accuracy for the early diagnosis of this condition [22,32].
Some patients (up to 10% [18]) may develop bronchiolitis
obliterans and organizing pneumonia (BOOP). The patients typically
present with more rapid onset hypoxemia. Chest radiographs in
these patients will reveal areas of consolidation and patients
respond to high dose corticosteroid administration with clearing
of the radiographic abnormalities [3].
Neutrophilic reversible allograft dysfunction:
Patients with NRAD have presumed BOS, but demonstrate improvement
in FEV1 following treatment with azithromycin [35]. These patients
have excess BAL neutrophils (≥ 15%) and no evidence of infection
[36]. CT imaging will demonstrate predominant centrilobular
nodules and tree-in-bud opacities [35].
Upper lobe fibrosis:
Upper lobe fibrosis has recently been described as a rare late complication of lung transplantation [31]. It may represent a form of chronic rejection [31].
Airway Complications:
For lung transplantation a systemic arterial supply is not established at the time of surgery. Viability of the anatomosis depends upon collateral flow from the pulmonary circulation. Arterial circulation to the bronchial anastomosis is usually not restored until approximately 4 weeks following transplantation. For end-to-end anastamoses, the use of an omental, pericardial, or intercostal muscle anastomotic wrap in the early post-operative period has reduced the incidence of ischemic induced airway necrosis and dehiscence. In single lung recipients the omentum is tunneled into the chest from the abdomen and will appear as a perihilar density. In double lung patients it is most often seen as a lower right paratracheal mass, as a convexity over the left upper cardiac margin, or in the left paraspinal region [3]. More recently many institutions have switched to a procedure which does not require a wrap procedure that uses a telescoping anastomosis (wrapping with pericardium or some other tissue is still sometimes performed [12]). In this procedure the membranous (or outer) portion of the donor bronchus is sutured end to end to the recipient bronchus, but the cartilagenous inner portion is inserted into the recipient bronchus for one or two cartilagenous rings. The internal margin of the anastamosis is not sutured and may result in an endolumenal flap [1,6].
1. Bronchial Dehiscence:
Bronchial dehiscence is the most common airway anastomotic complication in the early postoperative period (usually within the first month [33])- it occurs in 2-3% of cases. Ischemia at the anastomotic site is the major factor in development of this complication. Dehiscences smaller than 4 mm usually resolve without complication and do not require surgery [1]. Dehiscence is probably best assessed by bronchoscopy, however, CT will typically demonstrate the presence of extraluminal pockets of air around the anastamosis which is 100% sensitive and 72% specific for dehiscence. A small volume of extralumenal air can be present in the early post-operative period (up to 2-3 weeks), but there should be no free air by 4 weeks [34]. The actual bronchial defect may also be identified by MDCT. Confusion may arise in patients who have undergone a telescoping anastamosis in which an endolumenal flap is identified. In these cases the flap is always smooth, corresponds to the expected length of the invaginated bronchus (one to two ring lengths), and the air extends only to the outer margin of the anastomosis. These flaps are not seen along the posterior margin of the anastomosis (which is sutured). Patients with telescoping anastomoses may also develop small anastomotic divertiula which appear as smooth, rounded air collections at the inferior-medial aspect of the anastomosis [6].
2. Stricture/Bronchomalacia:
Stricture and bronchomalacia are usually seen within 4 months of transplant [33]. Anastomotic stricture (defined as a reduction of more than 50% in bronchial diameter [33]) occurs in about 10-15% of cases and the risk for stenosis may be increased with a telescoping anastamosis. The stenosis can occur at the anastomosis or in the central airways distal to the anastomosis [34]. The stenosis may be the result of ischemic injury after division of the branchial artery [34]. Stenosis in the central bronchi distal from the anastomosis can be found in up to 75% of cases and it is generally related to local ischemic [34]. Stenoses often manifest with progressive airflow obstruction that can be difficult to differentiate from other causes such as bronchiolitis obliterans syndrome. Treatment is with bronchoscopic dilatation or stenting, typically with an expandable metallic stent [1,34].
Stricture is probably best evaluated by bronchoscopy, however, CT will often demonstrate the area of narrowing. One must exercise caution in assessing bronchial caliber by axial CT- if the scan plane is too high or too low with respect to the lumen of the bronchus, the lumen can appear falsely narrowed. Helical CT with in-plane reconstructions will likely aid in detection of anastamotic strictures. Virtual bronchoscopy can also be used to demonstrate the stricture, but it is not 100% accurate and cannot detect all mucosal abnormalities [19].
Bronchomalacia is characterized by excessive airway collapse due to abnormal weakness of the airway walls and cartilage and flaccidity of the membranous portion of the trachea [34]. It is diagnosed by airway collapse or narrowing of 50% or more in cross-sectional area with expiration [33].
Vascular Complications:
Stenoses at vascular anastomoses are uncommon (fewer than 4% of cases) and are more common at the arterial anastomosis, than the venous anastomosis [1]. Pulmonary embolism occurs in up to 12-28% of patients following lung or heart-lung transplant- typically within the first 4 months. Most emboli occur within the allograft [33]. Up to 40% of affected patients can develop infarction [34]. The risk of pulmonary infarction is greatest in the immediate post-operative period because the newly transplanted lung does not have an alternate bronchial circulation [10].
Miscellaneous Complications:
Diaphragmatic dysfunction due to phrenic nerve paralysis is uncommon (fewer than 4% of cases). [1]
Infection:
Infection has been the leading cause of death in lung transplant recipients [10]. Factors that increase post-transplant patient's susceptibility to infection include immunosuppression, reduced mucociliary clearance, decreased cough reflex due to denervation, and interruption of lymphatic drainage. Risk factors for early post-operative infection include excessive duration of donor organ ischemia (over 72 hours), decreased donor arterial oxygen tension prior to harvesting (less than 350 mm Hg), recipient age over 40 years, positive donor sputum culture, and prolonged ventilatory support [33]. Although infection most commonly occurs in the transplanted lung, it is also the most common complication to affect the native lung (seen in 9% of patients- most commonly bacterial or fungal) [24].
1. Bacterial/Viral pneumonia:
Bacterial pneumonias are the most common infection following lung transplantation [17] and occur in greater than 35-75% of patients within 3 to 12 months post-transplant (highest incidence is during the first month post-transplant [33]). Bacterial pneumonias remain a major infectious complication throughout the transplant recipients life [23]. The donor lung is more commonly affected. The increased incidence of infection is likely multifactoral and related to immunosuppression, interruption of the lymphatic drainage, impaired mucociliary clearance, and denervation with impaired cough reflex. Bacterial infection is typically not often as lethal as viral or fungal infection. Organisms are mostly gram negative especially Enterobacter, Pseudomonas, and Klebsiella species [33]. Bronchitis secondary to Pseudomonas or Staphylococcus aureus can also be seen. Bacterial pneumonia typically manifests radiographically as a lobar or multilobar consolidation.
The incidence of pulmonary tuberculosis after lung transplantation is low (estimated to be between 2-3.8%) [23].
Viral pneumonias develop in about 11% of lung transplant patients [21] and occur at any time following transplantation. Infection with parainfluenza or respiratory syncytial virus is usually associated with symptoms of upper respiratory illness, no radiographic abnormalities, and a favorable prognosis [21]. Adenovirus infection usually produces acute symptoms of lower respiratory illness, produces radiographic abnormalities (opacities/consolidation- initially confined to transplanted lung, but rapidly progress), and is associated with a very high mortality (60%) [21].
2. Opportunistic Infection:
Opportunistic infections are also common after lung transplant surgery (34-59% of patients), but the infections do not seem to affect overall patient mortality.
CMV is the second most common cause of pneumonia in lung transplant patients, and it is the most common opportunistic infection (35-60% of opportunistic infections). It is the most significant viral infection and it usually occurs one to 4 months post-transplant. It is rarely seen earlier than 2 weeks after transplant [33]. CMV infection can take on one of three forms. Primary infection is the most serious and is seen in between 50 to 100% of seronegative patients that receive grafts from a seropositive donor. Secondary CMV infection develops from reactivation of latent disease in seropositive patients following institution of immunosuppressive therapy or from infection with as different strain of CMV. Secondary infection is usually less serious than primary infection. Infected patients may be asymptomatic or develop a fulminant pneumonia, possibly with extrathoracic findings such as retinitis, hepatitis, and gastritis. Presenting symptoms include dyspnea, fever, and cough. The diagnosis of CMV pneumonia can be made by bronchoscopy with lavage and biopsy [12]. Prophylactic therapy with acyclovir and immune globulin have not reduced the incidence of CMV infection in post-transplant patients. Prophylactic therapy with ganciclovir may reduce the incidence of CMV infection, and infection which occurs following discontinuance of the prophylaxis is often less severe. In single lung transplant recipients, the infection affects the transplanted side almost exclusively, with sparing of the contralateral native lung. The CXR will demonstrate no evidence of infection in about one-third of cases. Radiographic abnormalities almost exclusively involve the allograft [33]. The most common CXR finding in CMV infection is diffuse parenchymal haziness. Small ipsilateral pleural effusions are found in 20% of cases. CT findings in CMV infection include areas of ground-glass attenuation, reticulation, multiple, small ill-defined 1-3 mm nodules, tree-in-bud opacities [33], and even less commonly, areas of dense consolidation. [1,3,7,10].
Other less common causes of viral infections include herpes simplex virus. Patients with HSV infection present with fever, cough, and dyspnea, but will demonstrate symptomatic improvement after therapy with IV acyclovir. Radiographic findings may be absent, or demonstrate diffuse ground-glass opacities. Community acquired viral infection occurs in 8-14% of patients- particularly RSV, parainfluenza, adenovirus, and influenza [33]. Most of these viral infections occur from 2 weeks to 2 years after transplant and are considered a significant risk fator for development of bronchiolitis obliterans [33].
Fungal opportunistic infections are less common than viral infection, but are associated with a higher mortality. Fungal pneumonias usually occur between 10 and 60 days following transplant and more commonly involve the transplanted lung [23]. The most common findings for fungal infection on CT are a combination of nodules (multiple, varying sizes, and irregular margins), consolidation, and ground-glass opacification [23]. Pleural effusion is also common (63% of cases) [23].
Locally invasive or disseminated aspergillus infection accounts for 2-33% of post-transplant infections and 4-&% of lung transplant deaths [23]. Aspergillus infection is most commonly characterized by local invasion of a necrotic bronchial anastamosis (ulcerative tracheobronchitis)- typically found within 4 months of transplantation. Patients with Aspergillus airway colonization in the first 6 months after transplant are 11 times more likely to develop invasive disease than those not colonized during this period [23]. The CXR typically demonstrates no evidence of a change from baseline in these patients. The infection may progress to an invasive parenchymal disease at which time radiographic manifestations will become evident. Candida frequently colonizes the airways, but invasive pulmonary infection is uncommon.
There is an increased susceptibility to PCP infection, but prophylaxis with trimethoprim-sulfamethoxazole is effective in preventing the infection (incidence nearly 0%). Without prophylaxis the incidence of PCP infection approaches 90%.
Post transplantation Lymphproliferative Disorders and other malignancies:
(See also "Intrathoracic Lymphoproliferative Disorders")
Organ transplant patients are at increased risk for developing lymphoproliferative disorders ranging from benign polyclonal hyperplasia to malignant non-Hodgkins lymphoma- most are B-cell type. The disorders tend to occur within 1 year after transplantation (peak 3 to 4 months post-transplant). Definitive diagnosis requires pathologic confirmation [3]. PTLD develops in 4% to 10% of lung transplant patients which is slightly higher than the roughly 2% incidence in other solid organ transplants. The incidence of PTLD following lung transplant is probably higher in children [11,13]. Most cases of PTLD have been associated with concomitant Epstein-Barr virus infections (Epstein-Barr virus is found in about 90% of patients with PTLD [33]) and this may be etiologic agent. In patients receiving lung transplants, PTLD is more common in Epstein-Barr virus-seronegative recipients who receive EBV-seropositive donor lungs [1]. Epstein-Barr virus stimulates a B lymphocyte proliferation which is unopposed due to a cyclosporin induced inhibition of T-lymphocyte proliferation. The spectrum of post-transplant lymphoproliferative disease ranges from a premalignant hyperplasia to an aggressive high grade lymphoma. Treatment consists of a decrease or cessation of immunosuppressive therapy (cyclosporin) and the administration of antiviral agents (acyclovir). Regression occurs in 23% to 61% of patients after immunomodulation [13]. A potential complication of reducing immunosuppression is allograft rejection with bronchiolitis obliterans which can occur in up to 31% of patients [13].
Patients with PTLD may be asymptomatic, or complain of nonspecific complaints such as fever, weight loss, dyspnea, and lethargy [11]. Following lung transplant, PTLD is most commonly isolated to the lung. Patients that present with a solitary pulmonary nodule have a better overall prognosis [13]. PTLD's which occur early following transplantation tend to have a better prognosis, but this may be controversial [11]. About 15% of cases of PTLD may actually involve a T-cell proliferation. T-cell PTLD tends to occur later, not be associated with EBV infection, and is associated with a worse prognosis
For post-lung transplant PTLD, thoracic disease can be found in up to 69% of patients, which is much higher than PTLD associated with other solid organ transplants [13]. Solitary or multiple pulmonary nodules ranging in size from 1-2 mm to 5 cm are the most common pulmonary manifestation in cases of PTLD. A faint halo ground glass attenuation surrounding the nodules may be seen [11]. Mediastinal and hilar adenopathy can also been seen in 22% to 50% of cases. The lesion less commonly appears as patchy airspace or dense mass-like consolidation. Pleural effusions may occur. Following therapy, the nodules may resolve, leave a residual scar, or less commonly cavitate [13].
Differential considerations include infection (bacterial or fungal)- especially Aspergillus or Norcardia. These infections tend to cavitate and have an upper lobe predominence [11]. Transbronchial biopsy may also produce parenchymal nodular densities.
Besides lymphoproliferative disorders, skin malignancies and bronchogenic carcinoma can also develop in transplant patients [30]. The risk for developing bronchogenic carcinoma in the remaining native lung is about 1% (but increases to 2% in patients with underlying emphysema and 4% in patients with underlying pulmonary fibrosis) [30].
OKT3 Toxicity:
OKT3 is an antibody that binds to circulating T lymphocytes causing them to be opsonized by the reticuloendothelial system. The agent can produce pulmonary edema in some patients, typically within 6 hours of administration.
Hyperinflation of the Native Lung:
Hyperinflation of the native lung can be seen in up to 50% of patients with emphysema treated with single lung transplant [24]. Severe hyperinflation of the native lung may adversely affect graft function by compressing the graft and increasing pulmonary vascular resistance. Such patients may have clinical improvement after native lung volume reduction surgery [24].
Recurrence of primary disease:
Sarcoid is the most commonly recurrent primary disease (recurrence rate of about 35%) [33]. Other diseases reported to recur include lymphangioleiomyomatosis, EG, giant cell pneumonitis, pulmonary alveolar proteinosis, and pulmonary capillary hemagiomatosis [33].
Images:
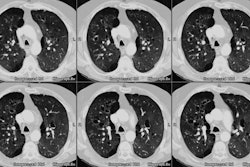
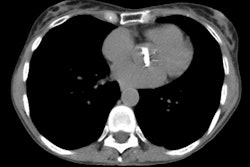
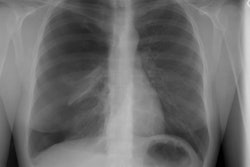
Case 1: Lung transplant for emphysema
REFERENCES:
(2) AJR 1995; 165(6): 1343-1348
(3) Radiol Clin North Am 1994; 32(4): 663-678
(4) ARRS 1996, Abstract #143
(5) AJR 1997; Collins J, et al. Ground-glass opacity at CT: The ABCs. 169: 355-367
(6) Radiology 1997; 203: 202-206
(7) Radiology 1996; 200: 349-356
(9) Radiology 1998; 206: 75-80
(10) RadioGraphics 1998;18: 21-43
(11) Radiology 1998; 206: 519-524
(12) Radiol Clin North Am 1998; 36 (1): 57-89
(13) AJR 1998; Pickhardt PJ, et al. Chest radiography as a predictor of outcome in posttransplant lymphoproliferative disorder in lung allograft recipients. 171: 375-382
(14) Radiology 1998; Lau DM, et al. Bronchiolitis obliterans syndrome: Thin-section CT diagnosis of obstructive changes in infants and young children after lung transplantation. 208: 783-788
(15) Thorax 1998; Habib SB, et al. Recurrence of recipient Langerhans' cell histiocytosis following bilateral lung transplantation. 53: 323-325
(16) Thorax 1998; Gabbay E, et al. Recurrence of Langerhans' cell granulomatosis following lung transplantation. 53: 326-327
(17) J Thorac Imaging 1998; Conces DJ. Pulmonary infections in immunocompromised patients who do not have acquired immunodeficiency syndrome: A systemic approach. 13: 234-246
(18) Chest 1998; Boehler A, et al. Bronchiolitis obliterans after lung transplantation. A review. 114: 1411-1426 (No abstract available)
(19) Radiology 1998; McAdams HP, et al. Bronchial anastomotic complications in lung transplant recipients: Virtual bronchoscopy for non-invasive assessment. 209: 689-695
(20) J Thorac Imaging 1999; Kundu S, et al. Correlation of chest radiographic findings with biopsy-proven acute lung rejection. 14: 178-184
(21) Radiology 1999; Matar LD, et al. Respiratory viral infections in lung transplant recepients: Radiologic findings with clinical correlation. 213: 735-742
(22) Radiology 2000; Lee ES, et al. Early bronchiolitis obliterans follwoing lung transplantation: Accuracy of expiratory thin-section CT for diagnosis. 216: 472-477
(23) AJR 2000; Collins J, et al. CT findings of pneumonia after lung transplantation. 175: 811-818
(24) Radiology 2001; McAdams HP, et al. Complications (excluding hyperinflation) involving the native lung after single-lung transplantation: Incidence, radiologic features, and clinical importance. 218: 233-241
(25) Radiology 2001; Bankier AA, et al. Bronchiolitis obliterans syndrome in heart-lung transplant recipients: Diagnosis with expiratory CT. 218: 533-39
(26) Radiology2001; Collins J, et al. Frequency and CT findings of recurrent disease after lung transplantation. 219: 503-509
(27) Radiology 2001; Siegel MJ, et al. Post-lung transplantation bronchiolitis obliterans syndrome: Usefulness of expiratory thin-section CT for diagnosis. 220: 455-462
(28) Radiology 2001; Gotway MB, et al. Acute rejection following lung transplantation: Limitations in accuracy of thin-section CT for diagnosis. 221: 207-212
(29) Radiology 2001; Marom EM, et al. Reperfusion edema after lung transplantation: Effect of daclizumab. 221: 508-514
(30) Radiology 2002; Collins J, et al. Bronchogenic carcinoma after lung transplantation: frequency, clinical characteristics, and imaging findings. 224: 131-138
(31) AJR 2003; Konen E, et al. Fibrosis of the upper lobes: a newly identified late-onset complication after transplantation. 181: 1539-1543
(32) Raduiology 2004; Konen E, et al. Bronchiolitis obliterans syndrome in lung transplant recipients: can thin-section CT findings predict disease before its clinical appearance? 231: 467-473
(33) Radiographics 2007; Krihnam MS, et al. Postoperative complications of lung transplantation: radiologic findings along a time continuum. 27: 957-974
(34) AJR 2008; Gill RR, et al. MDCT evaluation of central airway
and vascular complications of lung transplantation. 191: 1046-1056
(35) Radiographics 2017; Winningham PJ, et al. Bronchiolitis: a
practical approach for the general radiologist. 37: 777-794
(36) Semin Respir Crit Care Med 2013; Verleden SE, et al.
Neutrophilic reversible allograft dysfunction (NRAD) and
restrictive allograft syndrome (RAS). 34: 352-360