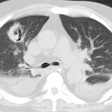
Mycobacterium Avium Complex:
Clinical:
MAC refers to M. avium and M. intracellulare infections which are the most common non-tuberculous mycobacterium to cause human disease. There are currently more than 140 nontuberculous myobacterial species, at least 40 of which are associated with lung infection [15]. Definitive diagnosis of pulmonary nontuberculous myobacterial infection can be difficult because the organisms are often saprophytes and they may colonize airways rather than infect them [14]. The infection typically occurs in immune compromised hosts. However, infection can also be seen in patients with pre-existing lung disease, as well as in patients with normal immunity. Approximately one-third of patients with CT findings of bilateral bronchiectasis are reported to have non-tuberculous mycobacterial pulmonary disease [17]. Treatment is often difficult, requires prolonged multi-drug therapy, and cure is not always achieved [7].
MAC in HIV:
MAC infections occur in 30-40% of HIV patients, typically late in the course of their illness when there is severe immunologic impairment with CD4 counts below 50-100 cells/uL [14]. Pulmonary disease is only noted in about 5% of patients and it is often aggressive and cavitary [12]. Although it is insignificant as a pulmonary pathogen, there is usually widespread dissemination to the bowel, bone marrow, and reticuloendothelial systems. Mortality is high even with treatment [12]. It is important to note that pulmonary infection does not produce granulomas.
In a new group of HIV patients, soon after initiation of antiretroviral therapy a focal inflammatory MAC lymphadenitis occurs without bacteremia. This seems to affect patients with a low CD4 count. Patients present with fever and marked cervical, intraabdominal, and intrathoracic lymphadenopathy. Affected patients typically demonstrate a decrease in HIV viral load and an increase in CD4 count [9]. These patients have probably had a sub-clinical MAC infection made apparent by partial immune reconstitution [8,9].
Other causes of immune deficiency due to steroids, organ transplant, lymphoreticular malignancy, and severe combined immunodeficiency can also be associated with MAC infection [12].
MAC in immunecompetent patients:
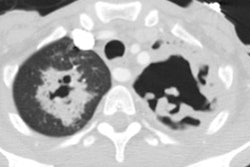
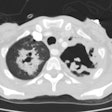
In the non-HIV population, approximately 50% of patients with MAC infection have pre-existing lung disease (such as chronic obstructive pulmonary disease (most commonly), bullous emphysema, healed prior TB, pulmonary fibrosis, or pneumoconiosis) and normal immunity. In these cases, patients are typically older (50-70 years) males. Patients typically present with a productive cough and insidious symptoms which are often attributed to worsening of their pre-existing lung disease [12]. Hemoptysis may occasionally be seen, and may be difficult to control. Systemic symptoms are rare. The infection typically manifests on CXR with features similar to reactivation TB. There is localized, upper lobe reticulonodular opacities (tree-in-bud) and thin walled cavities [12]. Occasionally there can be pleural thickening [12].
Another group of patients with MAC infection and normal immunity
has also been recognized consisting mostly of thin, white women
over the age of 55 years. It has been referred to as the "Lady
Windemere syndrome" due to the hypothesis that fastidiousness
among these women contributed to the development of the disease)
[12]. The course is variable, but tends to be chronic and indolent
[12]. Patients present with chronic cough, weight loss, and slowly
progressive radiographic abnormalities typically consisting of
"tree-in-bud" nodular infiltrates, multiple small well
circumscribed pulmonary nodules, and ground-glass opacities [12].
Bronchiectasis is found in a significant number of these patients
[12]. There is a predilection for the right middle lobe, lingula,
and dependent areas of the lungs [12]. In one study, without
treatment, 60% of patients with the nodular bronchiectatic form of
MAC lung disease showed disease progression and 40% showed stable
disease during a 28 month period (none showed spontaneous
improvement) [16].
The reported treatment success rate of MAC lung disease with
macrolide-containing regimens is approximately 60-80% (with
success defined as eradication of organisms without relapse over a
several year period after cessation of treatment [16]. The high
failure rate is caused by the prolonged treatment period, the side
effects of the antibiotic treatment, and possible reinfection
rather than relapse [16]. Following treatment, patients with the
nodular bronchiectatic form of MAC can demonstrate a significant
decrease in disease extent on CT [16].
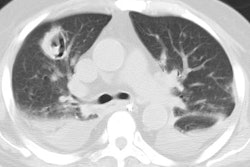
"Hot tub lung" is another form of the infection [12,13]. Patients are typically young (average age 40 years) and immunocompetent [12]. Patients typically have significant hot tube exposure (most often indoor tubs) for months to years [12]. Resolution of symptoms generally occurs with removal of the patient from the vicinity of the hot tub and treatment with antituberculous medication is usually not necessary [13]. CT scan shows ground-glass opacities (up to 75% of cases [13]), scattered nodules (ground-glass attenuation, diffuse, and centrilobular in up to 80% of cases [2]), and mosaic attenuation (secondary to air trapping associated with bronchiolitis) [12,13].
Certain musculoskeletal disorders such as pectus excavatum and scoliosis may also be associated with an increased risk for MAC infection.
X-ray:
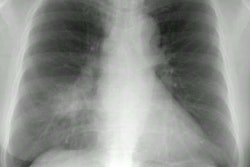
CXR findings are usually normal (in up to 21% of HIV patients) or non-specific such as a diffuse reticulonodular pattern associated with patchy alveolar infiltrates, adenopathy (common), or effusion.
The HRCT appearance of parenchymal disease is non-specific consisting of a bilateral small nodules, with or without areas of consolidation. Centrilobular opacities may also be identified. Pleural effusions are common, but miliary disease is rarely seen with MAC.
In non-immune compromised hosts, the HRCT findings include bilateral small nodules, cylindric bronchiectasis, and branching centrilobular opacities [10]. In fact, MAC is one of the most frequent causative organisms when bronchiectasis and bronchiolitis (tree-in-bud pattern) is identified involving more than one lobe- especially when there is associated lobular consolidation or a cavity [11].
REFERENCES:
(1) RadioGraphics 1997; Rubin SA. Tuberculosis and atypical mycobacterial infections in the 1990s. 17: 1051-1059 (No abstract available)
(2) Radiol Clin North Am 1994; Primack SL, Muller NL. High-resolution computed tomography in acute diffuse lung disease in the immunocompromised patient. 32 (4): 731-744 (p.738)
(3) Seminars in Respiratory Infections 1996; Rosenzweig DY. 11 (4) (December): 252-261 (Review. No abstract available.)
(4) ACR Syllabi #40:p.110,197-200
(5) Radiol Clin North Am 1994; Webb WR. High-resolution computed tomography of obstructive lung disease. 32 (4): 745-757 (p.753)
(6) Radiologic Clinics of North America 1997; McGuinness G. Changing trends in the pulmonary manifestations of AIDS. 35 (5): 1029-1082 (Review)
(7) Radiographics 1999; Erasmus JJ, et al. Pulmonary nontuberculous mycobacterial infection: Radiologic manifestations. 19: 1487-1503
(8) Society of Thoracic Radiology Annual Meeting 2000 Course Syllabus; Haramati LB. Emerging infections in AIDS. 117-119
(9) AJR 2000; Nalaboff KM, et al. Imaging of Mycobacterium avium-intracellulare infection in AIDS patients on highly active antiretroviral therapy: Reversal syndrome. 175: 387-390
(10) Radiology 2004; Jeong YJ, et al. Nontuberculous mycobacterial infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. 231: 880-886
(11) Radiology 2005; Koh WJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. 235: 282-288
(12) Chest 2006; Waller EA, et al. The expanding spectrum of mycobacterium avium complex-associated pulmonary disease. 130: 1234-1241
(13) AJR 2007; Hartman TE< et al. CT findings of granulomatous pneumonitis secondary to mycobacterium avium-intracellulare inhalation: "hot tub lung". 188: 1050-1053
(14) AJR 2007; Martinez S, et al. The many faces of pulmonary
nontuberculous myobacterial infection. 189: 177-186
(15) Radiology 2012; Kim HS, et al. Serial CT findings of
mycobacterium massiliense pulmonary disease compared with
myobacterium abscessus disease after treatment with antibiotic
therapy. 263: 260-270
(16) AJR 2013; Lee G, et al Serial CT findings of nodular
bronchiectatic Mycobacterium avium complex pulmonary
disease with antibiotic treatment. 201: 764-772
(17) AJR 2019; Choi Y, et al. Unilateral lung involvement of
nodular bronchiectatic Mycobacterium Avium complex
pulmonary diseases: proportion and evolution on serial CT studies.
212: 1010-1017